Red Cross starts vax for 5 to 11 years old
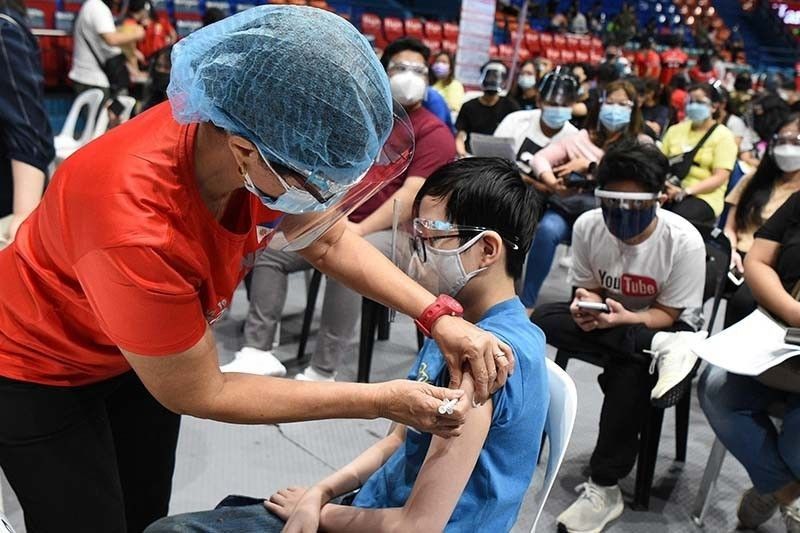
MANILA, Philippines — The Philippine Red Cross has started vaccinating against COVID-19 children aged five to 11, PRC chairman and Sen. Richard Gordon said yesterday.
In a statement, Gordon said the inoculation was kicked off initially in the cities of Mandaluyong, Tarlac and Zamboanga as well as in the province of Pangasinan.
“It is important that our children aged five to 11 years will not be left out in our vaccination efforts against this pandemic. Even if our cases are decreasing, it is still risky for them not to be vaccinated,” Gordon said.
He warned that children who would contract COVID would have “both short and long-term health complications.”
The Department of Health provided the PRC 3,000 doses of reformulated Pfizer vaccine for the five to 11 age group, Gordon said.
He gave assurance that the PRC would continue to deploy and operate its Bakuna Buses and Bakuna Centers, respectively, to help the government in ramping up inoculation efforts across the country.
Since March last year, when PRC started its vaccination program, it has administered over one million doses of vaccines through its 17 Bakuna Buses, 26 Bakuna Centers and Bakuna teams.
Meanwhile, Health Secretary Francisco Duque III said the fourth phase of the National Vaccination Days (NVD-4) for senior citizens is extended until March 18.
Duque said the extension would allow more senior citizens to avail themselves of the vaccines, noting they are the most vulnerable to the virus.
“It will be extended for our seniors until March 18. For other age groups, it ends (today),” Duque said
The NVD-4 was originally set from March 10 to 12 due to low coverage.
The target was to inoculate 1.8 million people, but as of March 14, only around 1.16 million or 62 percent have received the vaccines.
Duque also confirmed that Sinovac’s CoronaVac is now allowed for minors aged six to 17 years.
He said this is a welcome development as the supply of Pfizer vaccine for five to 11 years old is still limited.
The Food and Drug Administration approved the application of Sinovac’s local partner IP Biotech Inc. to amend its emergency use authorization for CoronaVac.
- Latest
- Trending































