COVID-19 vaccines may be commercially out this month
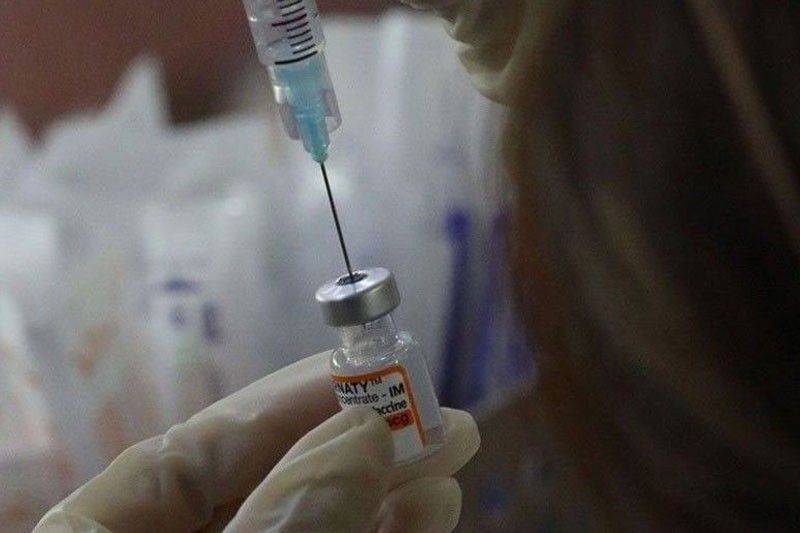
MANILA, Philippines — COVID-19 vaccines could soon become commercially available in the country as the release of the certificate of product registration (CPR) is anticipated later this month.
Dr. Rontgene Solante, a member of the Vaccine Experts Panel, revealed this during an interview on “The Chiefs” broadcast on Cignal TV’s One News channel Thursday night.
“I’ve heard it will be coming out soon, any time this June or early July. Hopefully, we will already have that (CPR),” Solante said.
He further explained that pharmaceutical companies had submitted applications for the CPR as early as last December or January. Considering the typical timeline for FDA approval, which is around six months for regular CPR, Solante believes the approval should have already been granted.
A CPR is a license granted by the FDA for the manufacture, distribution and sale of a medical device.
All COVID-19 vaccines and medicines in the government’s inventory are currently distributed and administered under an emergency use authorization or EUA.
“The requirement for CPR compared to a requirement for EUA is totally different,” explained Solante. “Much is being asked when you apply for CPR because it means you are selling it, it is for commercial use already.”
He cited, for example, one of the localization requirements for CPR issuance such as labeling. Illustrating his point, he said: “With the EUA, the Pfizer vaccine used in the United States is the same vial we used here in the country, there are no changes that come with it. But when you will be getting the CPR, there is a need to change it. The labeling now should be Philippine-recommended labeling.”?These are likely to take time as manufacturers comply with the technicalities. “But I really hope it will soon be over, and we can get approval to be able to have COVID-19 vaccines that include the bivalent vaccines,” Solante said.
- Latest
- Trending