Pfizer sees vaccine by September; Remdesivir proves effective
MANILA, Philippines — Pfizer Inc. has announced it could have a coronavirus vaccine ready by the fall, according to a report in The Daily Mail.
Pfizer chief executive officer Albert Bourla said on Tuesday a coronavirus vaccine for emergency use could be ready by the autumn and for broader roll out by the end of 2020.
It has already started testing the vaccine on humans in Germany with its partner firm BioNTech and hopes to begin testing in America soon.
The company has already started mass manufacturing doses while trials are underway and is aiming to have “hundreds of millions of doses ready for the end of the year.”
Developing vaccinations usually takes many months or years but researchers are hurtling towards human trials. They say the process has been made easier because the virus is not mutating and is similar to other viruses seen in the past.
As many as 100 potential COVID-19 candidate vaccines are now under development by biotech and research teams around the world, and at least five of these are in preliminary testing in people in what are known as Phase 1 clinical trials. Speaking to The Wall Street Journal, Bourla stated: “This is a crisis right now, and a solution is desperately needed by all.”
Meanwhile, the promise of an effective treatment against the coronavirus – an experimental drug that can speed the recovery of COVID-19 patients – raised hopes yesterday for faster progress in battling the pandemic and restoring wrecked economies and livelihoods.
The US government and others say they are working to make the medication available to patients as quickly as possible. News of the major medical advance lifted world markets, outshining gloomy data showing the US economy contracted nearly 5 percent in January-March in the worst downturn since the Great Recession.
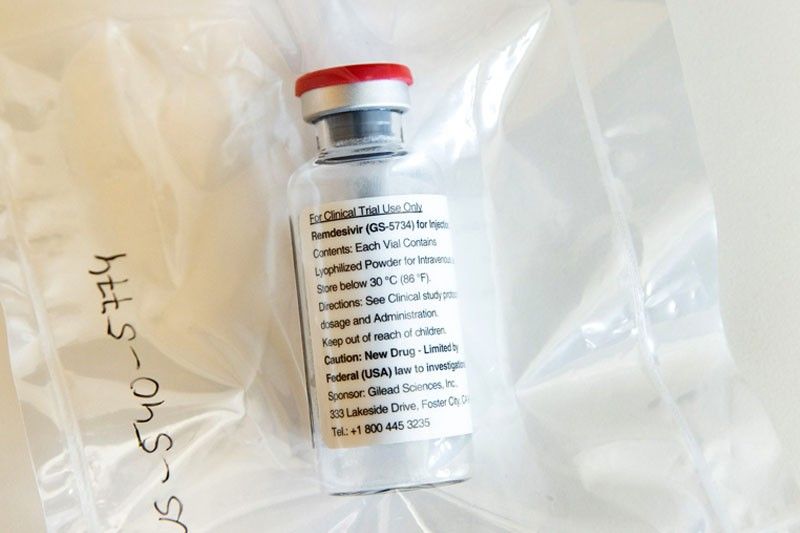
California-based biotech company Gilead Sciences and the US government reported in a major study run by the US National Institutes of Health that the drug remdesivir shortened the time it takes for COVID-19 patients to recover by four days on average, from 15 days to 11.
The study, involving 1,063 coronavirus patients around the world, also showed a trend toward fewer deaths among those on the drug, said Dr. Anthony Fauci, the government’s top infectious-disease expert.
“What it has proven is that a drug can block this virus,” he said. “This will be the standard of care.”
With a vaccine perhaps a year or more away, experts say an effective treatment could have a profound effect on the outbreak. Stocks surged around the world on the news, with the Dow Jones Industrial Average gaining more than 530 points on Wednesday, or over 2 percent.
The virus has killed over 220,000 people worldwide, including more than 60,000 confirmed deaths in the US, and led to lockdowns and other restrictions that have closed factories and other businesses around the globe.
Confirmed infections globally have reached about 3.2 million, including 1 million in the US, according to a tally by Johns Hopkins University. The true numbers of deaths and infections is likely much higher because of limited testing, differences in counting the dead and concealment by some governments.
The US said its gross domestic product, or output of goods and services, shrank at an annual rate of 4.8 percent in the January-March period, the sharpest quarterly drop since the global financial meltdown of more than a decade ago. That was before major shutdowns in many places.
And the worst is yet to come: The Congressional Budget Office has estimated the economy will shrink at a 40 percent annual rate in this quarter.
The latest figures on Americans applying for unemployment benefits came out yesterday, with economists estimating perhaps 1 in 6 workers, or nearly 30 million people, have lost their jobs over the past six weeks.
Mario Franco, who worked at a McDonald’s at a rest stop along Interstate 95 in Darien, Connecticut, for 26 years, rising to night manager in charge of the kitchen staff, was laid off in late March. The 50-year-old said he has little savings and now relies on a food bank and union donations.
“They didn’t give us any notice,” he said through an interpreter. “They didn’t tell us about it. Just suddenly the night shift ended and that was it. There was no more work.”
The US unemployment rate for April is due late next week, and economists have said it could range as high as 20 percent – a level last seen during the Depression.
Worldwide, the International Labor Organization, the UN labor body, forecast the pandemic has left 1.6 billion people depending on day labor, gig jobs and other informal work in immediate danger of losing their livelihoods. That is nearly half the global workforce of 3.3 billion.
Pushing to reopen the country, President Donald Trump was allowing federal social distancing guidelines to expire yesterday and even saying he plans to travel to Arizona next week.
Trump has laid out plans for returning to pre-virus normalcy despite doctors’ warnings that the country needs to embrace extended social distancing and mask wearing. The US virus death toll surpassed his forecast for 60,000 on Wednesday.
Authorities in some areas are opting for greater caution.
A memo sent to California police chiefs says Gov. Gavin Newsom will order all beaches and state parks closed starting Friday to curb the spread of the coronavirus.
Nevada’s Gov. Steve Sisolak said he was extending his directive asking people to stay at home until May 15 but easing restrictions on some outdoor activities and some businesses starting Friday.
China reported that its factory activity weakened in April as outbreaks clobbered global consumer demand, hindering efforts to revive the world’s second-largest economy. Surveys by a Chinese magazine and an official industry group showed activity slipped back after rebounding in March following the closure of much of China’s economy to fight the virus.
In Europe, almost every measure of the economy is in free fall. Figures due to be released Thursday are expected to show a drop of about 4 percent in the first three months of the year in the eurozone. An even steeper hit is projected for this quarter, and unemployment is expected to rise to about 8 percent.
Many economists are skeptical the US economy will bounce back quickly later in the year, noting that the virus could flare up again or consumers and employees might be too worried to return to business as usual.
“The virus has done a lot of damage to the economy, and there is just so much uncertainty now,” said Mark Zandi, chief economist at Moody’s Analytics.
Health Undersecretary Maria Rosario Vergeire said the Philippines is preparing to participate in another clinical trial to find an effective vaccine against COVID-19.
Vergeire said the Department of Health (DOH) is undertaking the necessary preparation for the trial for Avigan, a vaccine for influenza.
“We have expressed interest to take participate and we are waiting if the Philippines will be among the countries to receive the first batch of clinical trial to be launched by Japan in collaboration with other countries,” Vergeire said.
Vergeire said the Philippine General Hospital (PGH) is studying the protocol for the clinical trial.
The DOH, she said, is also doing the necessary preparations for the clinic trial while waiting for the arrival of vaccine from Japan.
Coconut oil
Science and Technology Secretary Fortunato dela Peña said that another study by the University of the Philippines-PGH (UP-PGH) will start next week to check the efficacy of virgin coconut oil (VCO) on patients infected with the coronavirus disease 2019 (COVID-19).
Dela Peña, in the Laging Handa morning virtual press briefing, said that the UP-National Institutes of Health (UP-NIH) team is just waiting for the approval of the ethics board of the UP-PGH for the start of clinical trials on COVID patients.
He earlier reported that the Department of Science and Technology (DOST) is providing a P5-million grant to two separate studies to be conducted to check if VCO could cure COVID-19.
The UP-PGH study on VCO titled “Virgin Coconut Oil and Omega-3a Adjunctive Therapy for Hospitalized Patients with COVID 19,” will be headed by Dr. Marissa Alejandria, the president of the Philippine Society for Microbiology and Infectious Diseases.
The other study is to be conducted by the DOST-Food and Nutrition Research Institute, the DOST-Calabarzon, in collaboration with the government of Santa Rosa, Laguna headed by Mayor Arlene Arcillas, the Philippine Coconut Authority (PCA) and Medical City South Luzon that would involve 90 persons under investigation (PUIs) for COVID-19 infection who exhibit symptoms of the virus.
The DOST study was approved by the DOST-FNRI ethics board last week.
Dela Peña said he expects the UP-PGH ethics board to also issue an approval soon.
An ethics committee review is required for the conduct of such clinical trials, Dela Peña said.
“All researches that involve living subjects require ethics clearance. Even animal trials. More so for humans,” he said.
Dela Pena said that the DOST study had enrolled 90 COVID-19 PUIs for the clinical trials.
Aside from the two VCO studies, the DOST is also funding another research on VCO by Prof. Fabian Antonio Dayrit of the Ateneo de Manila University in partnership with the Duke University-National University of Singapore. – Mayen Jaymalin
- Latest
- Trending