RITM to assess reliability of FDA-approved rapid test kits
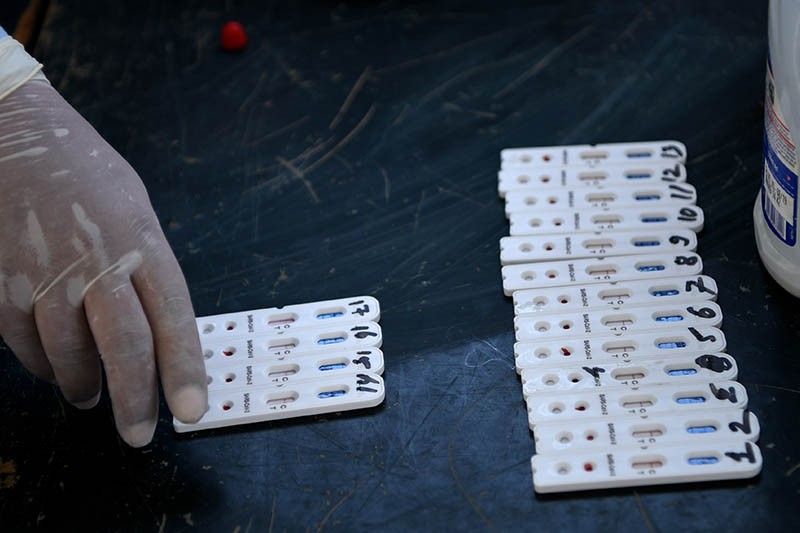
MANILA, Philippines — The Research Institute for Tropical Medicine will evaluate the reliability of approved rapid antibody test kits for the new coronavirus as the country boosts its testing capacity.
In a statement Tuesday, Food and Drug Administration Director General Enrique Domingo said the evaluation of RITM, the national reference laboratory for emerging diseases, will guide the public in selecting the kits they will use for testing.
The FDA has so far approved 16 antibody rapid tests.
“Recent news from other countries reporting poor performance of rapid antibody tests have cast doubts on the accuracy of some kits,” Domingo said.
“As the Philippines embarks on a mass testing strategy using both the polymerase chain reaction based kits and rapid antibody test kits, validation of rapid kits by the RITM would be helpful in choosing the best products to use as we go forward now and after the end of the enhanced community quarantine,” he added.
Rapid antibody tests detect the presence of antibodies in the blood of people believed to have contracted COVID-19. Some medical experts do not recommend the use of rapid test kits because of potential false positives and false negatives.
The World Health Organization also said it “does not recommend the use of antibody-detecting rapid diagnostic tests for patient care but encourages the continuation of work to establish their usefulness in disease surveillance and epidemiologic research.”
The government’s coronavirus response task force said rapid test kits must be used in conjunction with PCR-based test kits as the country began ramping up its testing efforts.
The national government is eyeing to conduct 8,000 tests daily by the end of April. To date, 58,072 individuals have been tested nationwide.
The new coronavirus has so far infected 6,599 people in the country, including 654 recoveries and 437 deaths.
- Latest
- Trending






























