Global COVID-19 vaccine race and related developments
As negotiations towards a new pandemic treaty pick up pace, observers warn of watered-down efforts to ensure equitable access to the medical products needed to battle future Covid-like threats.
Shaken by the pandemic, the World Health Organization's 194 member states are negotiating an international accord aimed at ensuring countries are better equipped to deal with the next catastrophe, or even prevent it altogether.
The process is still in the early stages, with the aim of reaching an agreement by May 2024.
But critics warn that revisions being made to the preliminary negotiating text are weakening the language -- notably in a key area aimed at preventing the rampant inequity seen in access to vaccines and other medical products during the Covid pandemic.
"I think it is a real step backwards," Suerie Moon, co-director of the Global Health Centre at the Geneva Graduate Institute, told AFP. — AFP
Africa's first mRNA vaccine hub is ceremonially launched on Thursday to acclaim from the UN's global health chief, who hailed it as a historic shift to help poor countries gain access to life-saving jabs.
The facility was set up in the South African city of Cape Town in 2021 on the back of the success of revolutionary anti-Covid vaccines introduced by Pfizer/BioNTech and Moderna.
"This precious project... will bring a paradigm shift in addressing the serious problem we faced, the equity problem, during the pandemic, so (that) it's not repeated again," World Health Organization (WHO) head Tedros Adhanom Ghebreyesus tells a media briefing to mark the inauguration. — AFP
China has approved its first locally developed messenger RNA (mRNA) vaccine against Covid-19, its manufacturer said Wednesday, months after the relaxation of strict Covid-zero regulations sparked a surge in cases.
The vaccine, developed by CSPC Pharmaceutical Group Ltd, has been approved for "emergency use" by Beijing's health regulator, the company said in a statement.
It showed high efficacy in a trial in which it was used as a booster shot for people who have been given other types of vaccines, the company added, without offering further details. — AFP
COVID-19 vaccine maker Novavax raises doubts about its ability to continue its business, announcing plans to cut spending after struggles in rolling out its coronavirus jab.
Shares of Novavax plummeted 25 percent in extended trading, after the company reported fourth-quarter earnings that missed analyst estimates.
While the firm should have enough money to fund operations, the situation is "subject to significant uncertainty," it says in a statement. — AFP
The protection against Covid-19 from being previously infected lasts at least as long as that offered by vaccination, one of the largest studies conducted on the subject says.
Ten months after getting Covid, people still had an 88% lower risk of reinfection, hospitalisation and death, according to the study published in the Lancet journal.
That makes this natural immunity "at least as durable, if not more so" than two doses of Pfizer or Moderna's vaccines, the study says.
The authors nevertheless emphasized that their findings should not discourage vaccination, which remains the safest way to get immunity. — AFP
Covax, the global programme for distributing Covid jabs to poorer countries, will soon be integrated into more routine vaccination programmes, Gavi said Thursday.
Gavi said its board agreed during a two-day meeting in Geneva this week to phase out Covax after 2023, stressing though that the Covid jabs would still be made available to less well-off countries, alongside other vaccines.
"While Covax continues to have in place plans for worst-case scenarios, the board agreed, in principle, to explore integrating future Covid-19 vaccinations into Gavi's core programming," it said in a statement.
The aim, it said, is "to improve synergies, be more responsive to countries' needs", and to reduce the current burden on countries of having a specialised emergency response in place. — AFP
The EU backs a Covid booster vaccine by French drug maker Sanofi and Britain's GlaxoSmithKline after it gave positive results against the Omicron variant in trials.
The approval of the "next generation" jab by the European Medicines Agency (EMA) is a shot in the arm for Sanofi and GSK, which have lagged behind rivals in offering a vaccine.
The VidPrevtyn Beta jab could be used as a booster in adults previously given mRNA jabs like the Pfizer/BioNTech and Moderna, or viral vector vaccines made by AstraZeneca and Johnson & Johnson, the EMA says.
"A booster dose of VidPrevtyn Beta is expected to be at least as effective as Comirnaty (Pfizer's vaccine) at restoring protection against Covid-19," the Amsterdam-based EMA says in a statement. — AFP
Pfizer-BioNTech says Thursday they will test a combined coronavirus and influenza vaccine, which could potentially pave the way for better inoculation uptake for both illnesses.
The companies say in a statement the mRNA-based combination vaccine candidate was set to progress to a phase one trial in the United States with 180 volunteers. — AFP
AstraZeneca's Covid vaccine has been linked to a 30-percent higher risk of getting a very rare blood clotting condition compared to the Pfizer jab, a large international study said Thursday.
Several countries have already altered their advice after previous research indicated that -- in a tiny number of cases -- thrombosis with thrombocytopenia syndrome (TTS) can be a possible side effect of Covid vaccines that use an adenovirus vector, or "engineered" virus, such as those from AstraZeneca and Johnson & Johnson.
Thrombocytopenia produces potentially life-threatening blood clots with low levels of blood platelets -- the small cell fragments in our blood that prevent bleeding.
The new study, published in the journal BMJ, was the first to compare thrombocytopenia rates between adenovirus and mRNA vaccines -- such as Pfizer -- across multiple countries. — AFP
Indonesia has approved its first locally developed Covid-19 vaccine for emergency use, the head of the country's public health agency says Friday, hailing it as a step toward "the nation's independence in access to medicine".
Jakarta has stressed the importance of developing national vaccines since the beginning of the pandemic but it currently relies on China's Sinovac and the Western-made Moderna and Pfizer-BioNTech mRNA jabs.
The IndoVac jab, developed by state-owned pharmaceutical company Bio Farma and Texas-based Baylor College of Medicine, can now be used as a primary dose for an unvaccinated or partially vaccinated adult in the world's fourth most-populous country.
"The development... of a domestic vaccine is a pride for us Indonesians as a foundation and as the first step to achieve the nation's independence in access to medicine," head of the national food and drugs agency (BPOM) Penny Lukito says at a press conference Friday. — AFP
North Korea will begin vaccinating its people against Covid-19 around November, state media said Friday, about a month after the country declared victory over the virus.
It is the first time the isolated regime officially announced its plans for vaccination since the start of the pandemic.
"Along with responsible vaccine administration, we need to recommend that all citizens wear a mask to protect their own health starting November," leader Kim Jong Un said, according to Pyongyang's official Korean Central News Agency (KCNA).
Health experts in the country believe North Koreans' antibody levels — acquired during the epidemic which the regime confirmed in May — will decrease by October, Kim added.
Friday's KCNA report did not elaborate on where the vaccines were from. — AFP
Moderna says it is suing rival vaccine makers Pfizer and BioNTech, alleging the partners infringed on its patents in developing their COVID-19 shot administered to hundreds of millions around the world.
The lawsuits set up a high-stakes showdown between the leading manufacturers of COVID-19 shots that are a key tool in the fight against the disease.
"Moderna believes that Pfizer and BioNTech's COVID-19 vaccine Comirnaty infringes patents Moderna filed between 2010 and 2016 covering Moderna's foundational mRNA technology," the US-based biotech firm says in a statement. — AFP
A panel of experts convened by the US Food and Drug Administration unanimously recommends Covid-19 vaccines for children under five, the final age group awaiting immunization in most countries.
Formal authorizations should follow soon, with the first shots in arms expected early next week, just over a year-and-a-half after the first Covid vaccines were greenlighted for the elderly in December 2020.
"This recommendation does fill a significant unmet need for a really ignored younger population," says Michael Nelson, a professor of medicine at the University of Virginia, one of the 21 experts asked to vote for the milestone meeting. — AFP
A panel of US medical experts recommends the Moderna COVID-19 vaccine for use in children aged six through 17.
Formal authorization should soon follow, at which point families will have a second option against the coronavirus, as Pfizer's vaccine was given the greenlight for teens and younger children last year.
After weighing available data, 22 experts convened by the US Food and Drug Administration unanimously agreed that the known benefits outweighed the known risks when Moderna's vaccine was administered as two shots at the adult dose of 100 micrograms to those aged 12-17, and half of that for children 6-11. — AFP
A panel of experts convened by the US drug regulator recommends the Novavax COVID-19 shot, a late runner in the fight against the virus that could nonetheless play a role in overcoming vaccine hesitancy.
Three vaccines are currently approved in the United States: Pfizer and Moderna, which are based on messenger RNA, and Johnson and Johnson, which recently received a recommendation against broad use becase of links to a serious form of clotting.
Experts voted 21 in favor of the Novavax vaccine, with none against, and one abstention, despite some concerns it may be linked to rare cases of heart inflammation. — AFP
The Food and Drug Administration voices concern about myocarditis being potentially linked to the Novavax COVID-19 vaccine, just as experts are to weigh its use in the United States.
The Novavax vaccine is already authorized in other countries, particularly in Europe. In the United States, an independent committee convened at the request of the FDA is to meet Tuesday to evaluate data from the clinical trials of Novavax and give its recommendation.
In advance of that, the agency published a lengthy document on Friday analyzing these results, as it had done for the three other vaccines already authorized in the country. — AFP
The head of US pharmaceutical giant Pfizer says a COVID-19 vaccine effective against multiple variants is possible before the end of 2022.
Chairman Albert Bourla says the firm was also working on producing a vaccine that could provide good protection for a whole year, meaning people would come back annually for boosters, as with influenza shots.
"I hope, clearly by autumn... that we could have a vaccine" that worked against not only the dominant Omicron but all known variants, he says. — AFP
Australia is offering a fourth dose of COVID-19 vaccines to over 65s from next month, federal health authorities announce, as a new Omicron strain races through the population.
The top advisory group on vaccines approved the fourth shot for vulnerable groups: those aged over 65, indigenous people over 50, people who are immunocompromised and care home residents.
In the past six weeks, other countries, including France, Germany and Sweden as well as health authorities in England, have recommended a fourth Covid-19 vaccine dose for the most vulnerable, including the elderly. — AFP
Moderna announces it had asked the United States drug regulator for emergency authorization for a second booster shot of the company's COVID-19 vaccine for all adults.
The request to the US Food and Drug Administration (FDA) would "allow for a fourth dose of our #COVID19 vaccine in adults 18 years of age and older who have received an initial booster" of any approved Covid jab, Moderna said on Twitter.
The request comes days after Pfizer-BioNTech, makers of the other Covid mRNA vaccine, also requested emergency approval for a second booster shot, but their request was limited to adults aged 65 and older. — AFP
Moderna announces it had asked the United States drug regulator for emergency authorization for a second booster shot of the company's Covid-19 vaccine for all adults.
The request to the US Food and Drug Administration (FDA) would "allow for a fourth dose of our #COVID19 vaccine in adults 18 years of age and older who have received an initial booster" of any approved Covid jab, Moderna says on Twitter.
The request comes days after Pfizer-BioNTech, makers of the other Covid mRNA vaccine, also requested emergency approval for a second booster shot, but their request was limited to adults aged 65 and older. — AFP
The United States has given more than 500 million coronavirus vaccines to other countries since the jabs were developed, Secretary of State Antony Blinken said Thursday in a statement obtained by AFP.
"The United States has now shared over 500 million safe and effective Covid-19 vaccine doses, free of cost, to more than 110 countries and economies around the world — for the sole purpose of saving lives," Blinken said.
Washington aims to more than double that amount to 1.1 billion doses, as the Covid pandemic persists around the globe. — AFP
The World Trade Organization chief hails a breakthrough between the EU, the United States, India and South Africa on waiving intellectual property rights on Covid-19 vaccines.
"This is a major step forward and this compromise is the result of many long and difficult hours of negotiations," Ngozi Okonjo-Iweala says.
"But we are not there yet. We have more work to do to ensure that we have the support of the entire WTO membership." — AFP
Pfizer and BioNTech announce they have formally asked the United States drug regulator for emergency approval of a second booster shot of their Covid vaccine for people aged 65 and older.
The companies say in a press statement that their request is based off two Israeli studies that show "an additional mRNA booster increases immunogenicity and lowers rates of confirmed infections and severe illness."
Most countries' case-levels have significantly declined from record levels during the Omicron wave, though multiple countries have seen levels plateau or start to tick up as they lift restrictions, and protection from prior doses begins to fade. — AFP
A UN human rights expert says the world should provide millions of doses of COVID-19 vaccines to North Korea, where "draconian" anti-pandemic measures are worsening an already-severe food crisis,
The impoverished nation has been behind a rigid self-imposed coronavirus blockade since early 2020 to protect itself from the pandemic, with the economy suffering and trade all but stopped.
The country's "draconian" anti-Covid measures, including border closures and further limits on domestic freedom of movement, have worsened the food crisis, Tomas Ojea Quintana, UN special rapporteur on human rights, says. — AFP
A White House official says the United States shipped nearly 5.2 million doses of the Pfizer COVID-19 vaccine to Egypt and Nigeria.
The shipments were the latest in a global campaign of donations from the United States. More than 400 million shots have already been dispatched from a target of 1.1 billion.
The official, who asked not to be named, says that 2,999,880 Pfizer doses were heading to Nigeria and 2,158,650 doses to Egypt. The shipments, which left Thursday and were due to arrive by Monday, went through Covax, the global distribution initiative co-led with public-private partnership Gavi. — AFP
All children aged five to 11 in England will be offered a Covid-19 vaccine, the UK government says Wednesday, following similar announcements in the rest of the UK.
However, Health Secretary Sajid Javid — who has responsibility for England only — says he had now accepted guidance from the Joint Committee on Vaccination and Immunisation, which advises UK health departments, to expand the rollout.
"The NHS (National Health Service) will prepare to extend this non-urgent offer to all children during April so parents can, if they want, take up the offer to increase protection against potential future waves of Covid-19 as we learn to live with this virus," he says in a statement. — AFP
The EU stands by its refusal to lift patent protections on Covid vaccines, just days ahead of a summit with African Union countries who see the issue as a priority.
The members of the African Union have pushed to include the demand in the conclusions of the joint EU-AU summit that starts on Thursday in Brussels.
"The African Union... urges the European Union to engage constructively towards the conclusion of a targeted and time limited waiver," says the AU proposal seen by AFP. — AFP
The US Food and Drug Administration announces it was postponing a meeting scheduled for next week where a panel of experts would have decided whether to endorse the Pfizer-BioNTech Covid vaccine for infants.
Both the FDA and Pfizer-BioNTech now say they want to wait for results of three doses among children aged six months through four years, rather than look to authorize a two-dose series -- meaning a final decision could take several more weeks.
"The companies expect to have three-dose protection data available in early April," the US and German pharmaceuticals says in a statement. — AFP
Johnson & Johnson has temporarily suspended production at a key plant manufacturing the Covid-19 vaccine, the New York Times reports.
The facility in the Dutch city of Leiden halted output late last year, according to the report, which cited people familiar with the decision.
J&J, without confirming or denying the report, says it has continued to fulfill delivery commitments, a company spokesman says. — AFP
Pfizer and BioNTech say they are seeking emergency authorization from US health regulators for use of their COVID-19 vaccine for children aged over six months and under five years.
If the Food and Drug Administration (FDA) authorizes the two-shot regimen, it will become the first COVID-19 vaccine available to this age group in the United States.
The companies say Tuesday that they started submitting their formal application "following a request" from the FDA, which seemingly wants to get the process moving quickly. — AFP
Covax aims to break the Covid-19 pandemic in 2022 by ensuring a steady supply of vaccines at last for the world's poorest countries — and swiftly getting them into arms.
The global scheme, aimed at procuring donor-funded jabs for the 91 weakest economies, delivered its one billionth dose last weekend — a major milestone that came far later than anticipated after a year of setbacks.
The battle for Covax in 2021 was getting hold of doses — besides rich countries cornering most of the vaccine supply, it faced export bans from producer countries, regulatory red tape and manufacturing delays. — AFP
Around a hundred Rwandans have crossed into DR Congo in recent days, saying they are fleeing the country's Covid-19 vaccination rules, local sources said on Wednesday.
Small groups of Rwandans, travelling by canoe, have landed on the southern edge of Idjwi island in Lake Kivu which straddles the border, Karongo Kalaja, the administrator of Idjwi, told AFP.
"We have already recorded at least 100" arrivals, Kalaja said. — AFP
India extends Covid vaccinations to teens aged 15-18 on Monday, after officials tightened restrictions in big cities to avoid a repeat of last year's devastating outbreak.
More than 200,000 people around India died in a huge spring virus wave that overwhelmed hospitals and crematoriums.
Health workers have since administered more than 1.4 billion vaccine doses but less than half of India's population is fully inoculated, according to government data. — AFP
A preliminary South African government study published Thursday showed a booster of the Johnson & Johnson COVIDvaccine was 85% effective in preventing hospitalization from the Omicron variant, a finding that helps revive the shot's reputation.
The South African Medical Research Council compared 69,000 health care workers who received two doses of the vaccine, based on viral vector technology, against a group of people who were unvaccinated.
The research, which has not yet been peer reviewed, was conducted from November 15 to December 20, a time when the heavily-mutated Omicron variant increased from 82 to 98% of COVID-19 cases in the country.
When a booster shot was given six to nine months after the first dose, vaccine efficacy against hospitalization increased over time, from 63% at 0-13 days to 85% one to two months post-boost.
"This data is important given the increased reliance on the Ad26.COV.2 vaccine in Africa," wrote the authors, using the formal name for the J&J shot.
The result was also hailed by the company. In a statement, J&J scientist Mathai Mammen said it showed the vaccine "remains strong and stable over time, including against circulating variants such as Omicron and Delta." — AFP
Chile will offer its citizens a fourth coronavirus vaccine dose from February, starting with high-risk categories, President Sebastian Pinera announces.
"The main concern and priority is to protect the lives and health of our compatriots," he says at an event to mark a year since Chile launched its COVID-19 vaccination campaign.
First to get the booster shot will be health workers, old people and those with chronic diseases. — AFP
AstraZeneca says third jab 'significantly' boosts antibodies against Omicron.
The EU's drug regulator says on Thursday it would decide whether the Novavax coronavirus jab will become the fifth vaccine approved for the bloc at a meeting next Monday.
The US firm's shot uses a more traditional technology than current vaccines, which experts hope could ease hesitancy and scepticism among the unvaccinated.
"Our human medicines committee will hold an extraordinary meeting on 20 Dec to review the application for the Covid-19 vaccine developed by Novavax," the European Medicines Agency says on Twitter on Thursday.
"We will communicate the outcome of this scientific discussion." — AFP
?? Our human medicines committee will hold an extraordinary meeting on 20 Dec to review the application for the #COVID19vaccine developed by #Novavax.
— EU Medicines Agency (@EMA_News) December 16, 2021
We will communicate the outcome of this scientific discussion.
The European Medicines Agency says Wednesday that Johnson & Johnson's Covid vaccine can be used as a booster shot two months after the first dose was administered, or after receiving other mRNA shots.
"EMA's human medicines committee (CHMP) has concluded that a booster dose of Covid-19 vaccine Janssen may be considered at least two months after the first dose in people aged 18 years and above," the watchdog says in a statement, sing the vaccine's commercial name.
This is the third vaccine after Pfizer/BioNTech and Moderna that the Amsterdam-based agency has approved for a booster for adults. — AFP
Russia admits that its homegrown Sputnik V coronavirus vaccine had not yet been approved by the World Health Organization because Russian authorities had not provided enough data.
In August last year, Sputnik V became the world's first approved coronavirus vaccine, when Russian authorities gave it the green light for domestic use ahead of large-scale clinical trials.
"We still haven't provided certain information that needs to be provided for certification because we had a different understanding of what information it had to be and how it should be provided," Kremlin spokesman Dmitry Peskov tells reporters. — AFP
A Canadian government immunization advisory committee urges COVID-19 booster shots for people 18 and older who are at greater risk of infection, while also strongly recommending those 50 years-plus to get the third jab.
The updated guidance comes after two provinces -- Ontario and Alberta, with half the population of Canada -- this week said they would start offering third jabs to people 50 and 60 years and over, respectively, and planned to further expand eligibility in the new year, amid concerns over the Omicron variant.
Eleven cases of the Omicron variant have so far been recorded in Canada, linked to travel abroad, public health officials say. — AFP
The WHO issued stern warnings Wednesday on the dangers of vaccination apathy and the European Union put mandatory jabs on the table as the United States registered its first case of the fast-spreading Omicron strain of the coronavirus.
The new variant, first reported to the World Health Organization by South Africa a week ago, has quickly popped up across continents, darkening economic forecasts and deepening fears of another difficult winter in the northern hemisphere.
"Globally, we have a toxic mix of low vaccine coverage, and very low testing — a recipe for breeding and amplifying variants," said WHO chief Tedros Adhanom Ghebreyesus, reminding the world that the Delta variant "accounts for almost all cases". — AFP
Pfizer announces it was seeking US authorization for Covid booster shots among adolescents aged 16 and 17, as concerns grow about the impact of the new Omicron variant.
The Food and Drug Administration (FDA) has so far only granted emergency use authorizations (EUAs) for boosters to people aged 18 and over, six months after their primary series of the Pfizer or Moderna Covid vaccine, or two months after the Johnson & Johnson shot.
"Today, we submitted a request to the @US_FDA to expand the emergency use authorization of a booster dose of our COVID-19 vaccine to include 16- and 17-year-olds," Pfizer CEO Albert Bourla wrote on Twitter. — AFP
Today, we submitted a request to the @US_FDA to expand the emergency use authorization of a booster dose of our COVID-19 vaccine to include 16- and 17-year-olds. It is our hope to provide strong protection for as many people as possible, particularly in light of the new variant.
— Albert Bourla (@AlbertBourla) November 30, 2021
Existing Covid-19 jabs will struggle against the Omicron variant and it will take months to develop a new shot that works, the head of US vaccine manufacturer Moderna tells the Financial Times.
Stephane Bancel tells the newspaper in an interview published Tuesday that data would be available on the effectiveness of current vaccines in the next two weeks but scientists were not optimistic.
"All the scientists I've talked to ... are like 'this is not going to be good'," he tells the newspaper. — AFP
Chile on Thursday announces it would start vaccinating children aged three and up against the coronavirus, after successfully innoculating around 90 percent of its initial target population.
Children under the new rollout will receive the Chinese CoronaVac shot already used for kids aged six to 15, the Public Health Institute says.
For 16 to 18-year-olds, Chile uses the Pfizer/BioNTech vaccine. — AFP
COVID-19 vaccines reduce transmission of the dominant Delta variant by about 40%, the WHO says, warning that people were falling into a false sense of security concerning jabs.
The World Health Organization's director-general Tedros Adhanom Ghebreyesus says many vaccinated people were wrongly thinking the jab meant they no longer needed to take any other precautions.
Fully-immunized people must stick with measures to avoid catching the virus and passing it on, Tedros insists, spelling out how the more contagious Delta meant the vaccines were not as effective against transmission. — AFP
Canada begins mmunizing children aged 5-11 against COVID-19, joining a handful of nations including Israel and the United States in offering shots to this age group.
At Montreal's convention center, a few dozen youngsters were among the first to receive the Pfizer doses authorized since last Friday for this age group.
To help ease their fears of needles, additional measures have been taken such posting stickers of unicorns or hockey players on partitions between nursing stations, longer appointments than for adults, and a dog to pet. — AFP
Leading Russian doctors on Wednesday invited celebrities and politicians with anti-vaccine views to visit COVID red zones in hospitals and see for themselves the effects of the pandemic.
In an open letter published by state news agency TASS, 11 doctors from several cities wrote to a dozen public figures who expressed anti-vaccine views to hundreds of thousands of followers on social media.
Russia, one of the countries worst-hit by the coronavirus pandemic, is struggling with widespread opposition to vaccination even though it has developed several homegrown jabs including Sputnik V.
Despite multiple pleas from President Vladimir Putin, only 37% of Russians are fully vaccinated and the country has seen more than 1,000 deaths a day in recent weeks.
In their letter, the doctors told several singers, actors, TV personalities and politicians who had expressed skepticism over vaccinations that they would take the time to show them around COVID treatment centers. — AFP
The United States is shipping another four million Covid-19 vaccine doses to Vietnam, the White House said Tuesday, bringing the total of US doses donated globally to nearly 270 million.
A senior administration official told AFP that 4,149,990 doses of the Pfizer vaccine are being sent, bringing the total delivered to Vietnam by the United States to 17,589,110 doses. Shipments began Tuesday.
Globally, there have now been 268,472,780 doses sent out to 110 countries, which the official, who asked not to be identified, said "is more than all countries combined have shared." — AFP
Germany says on Tuesday it expected to make Covid jabs mandatory for soldiers "soon", amid growing calls for the country to consider a general vaccine mandate to combat a raging fourth wave of the pandemic.
Defence ministry officials and army personnel representatives have agreed "to include the Covid vaccine on the list of vaccines" required for soldiers, a ministry spokesman tells AFP.
Although the mandate has yet to be formalised, "the implementation is expected soon", he adds. — AFP
Israel began rolling out Covid-19 vaccines for children aged five to 11 on Monday, becoming one of a handful of countries to inoculate children so young as it seeks to ward off another pandemic wave.
Over the summer, the Jewish state experienced an upsurge in coronavirus infections, fuelled by the Delta variant, and launched one of the earliest campaigns for booster shots.
As infections start to creep up again, Prime Minister Naftali Bennett has said the country is experiencing a "children's wave" with about half of the recently confirmed cases among children below the age of 11, he wrote on Facebook. — AFP
Iraq says it has received 1.2 million doses of Pfizer-BioNTech's Covid-19 vaccine through the Covax sharing scheme, amid fears of a fourth wave in the country.
Nearly seven million Iraqis have received at least one dose of coronavirus vaccine, amounting to 17.5% of the country's 40 million population, based on government figures.
Plagued by years of conflict, corruption and neglect, Iraq's health system has struggled to cope with the pandemic. — AFP
The United States authorizes the Pfizer and Moderna COVID-19 vaccine boosters for all people aged 18 and older on Friday, as the world's hardest-hit country enters a new winter wave of the pandemic.
Boosters were previously available to the immune compromised, people over 65, those at high risk of severe disease, and people in high risk occupations.
The new decision "helps to provide continued protection against Covid-19, including the serious consequences that can occur, such as hospitalization and death," acting commissioner Janet Woodcock of the Food and Drug Administration (FDA) says. — AFP
US Secretary of State Antony Blinken announces a deal to bring Covid-19 vaccines into conflict zones, where paltry numbers of people have been inoculated.
In a virtual ministerial meeting on the pandemic, Blinken says the United States had worked with Covax, the international vaccine alliance to support developing nations, on providing the single-dose Johnson & Johnson shots to areas of conflict and other humanitarian distress.
"We're eager for people in these difficult circumstances to get protection against Covid-19 as soon as possible," Blinken says. — AFP
The developer of Russia's coronavirus vaccine Sputnik V says Moscow should make jabs mandatory as inoculation rates remain low despite record deaths and campaigning by authorities.
His call came as Russia reported a record 1,239 Covid deaths in a single day Wednesday.
Only 34 percent of the country is fully vaccinated, even though Sputnik V -- the world's first Covid vaccine -- has been widely available since December last year. — AFP
Drugmakers Pfizer and BioNTech announces they have formally submitted a request asking US officials for emergency authorization of their Covid-19 booster vaccine for people aged 18 and older.
The move follows research published by the companies in late October indicating a third shot is 95.6 percent effective against symptomatic infection, based on clinical trials carried out on 10,000 people.
The companies asks the US Food and Drug Administration to add the new population segment, amending emergency use authorization already granted in September for a third dose for everyone aged 65 and up, as well as people at high risk of developing severe Covid-19. — AFP
Moderna has applied to the European Union's medicine regulator for approval of its Covid-19 vaccine for children aged six to 11, the US biotechnology company announces Tuesday.
The dosage for the two injections administered four weeks apart has been adjusted to 50 micrograms, compared with the 100 micrograms for shot recipients in older age groups.
"We are pleased to announce the submission of this variation to the EMA," the European Medicines Agency, Moderna's chief executive Stephane Bancel says in a statement. — AFP
US company Ocugen announces that it had asked authorities for emergency use authorization for COVID-19 vaccine Covaxin, which was developed in India, for ages 2 to 18.
Ocugen's data, gathered from clinical trials conducted outside of the United States with only a small group of children, may not be enough for the Food and Drug Administration (FDA) to grant the request.
Covaxin, developed in India by Ocugen's partner, Bharat Biotech, gained emergency approval from the World Health Organization on Wednesday and has already been cleared for use in 17 countries. — AFP
Moderna cuts its 2021 forecast for Covid-19 vaccine deliveries, pushing back some doses to next year, citing longer delivery times for international shipments. — AFP
Canada's most populated provinces say they will not force health care workers to get Covid vaccines, saying mass layoffs of staff who do not get shots would devastate hospitals.
The decisions by the governments of Ontario and Quebec — the provinces hardest hit by the pandemic — were announced separately.
Quebec had originally set a mid-October deadline for its health care workers to be fully vaccinated, but pushed it to back November 15 in hopes that more people might come around. — AFP
President Joe Biden hails US health authorities' approval for giving children aged 5-11 the Pfizer-BioNTech Covid vaccine as a "turning point" in the fight against the pandemic.
Vaccinating younger children will "allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus," Biden says in a statement released by the White House. — AFP
An expert panel unanimously recommends Pfizer-BioNTech's Covid vaccine for five- to 11-year-olds on Tuesday, the penultimate step in the process that will allow injections in young children to begin this week in the United States.
The Centers for Disease Control and Prevention (CDC), the top US public health agency, is expected to endorse that recommendation later Tuesday.
The Food and Drug Administration (FDA) approved the vaccine for the age group last week, but since it is distributed through public services, the CDC is also required to recommend it. — AFP
US biotech firm Moderna says that American officials have delayed approving its Covid-19 vaccine for teenagers to allow more time to better assess the potential risk of developing myocarditis, or heart inflammation.
The US Food and Drug Administration (FDA) on Friday "informed Moderna that the agency requires additional time to evaluate recent international analyses of the risk of myocarditis after vaccination," the biotech company says Sunday in a statement.
The evaluation on whether to recommend Moderna's vaccine for 12- to 17-year-olds could last until January 2022, the company says. — AFP
The United States authorizes the Pfizer COVID-19 vaccine for children aged five-to-11, paving the way for 28 million young Americans to soon get immunized.
The decision came after a high-level medical panel advising the government this week endorsed the shots, ruling that the known benefits outweighed the risks of side-effects.
The United States follows only a handful of other countries including China, Chile, Cuba and the United Arab Emirates that are inoculating younger children with various vaccines. — AFP
A medical panel of US government advisors vote to recommend authorizing the Pfizer Covid-19 vaccine for five-to-11-year-olds, paving the way for younger children to get their shots within weeks.
"It is pretty clear to me that the benefits do outweigh the risk when I hear about children who are being put in the ICU, who are having long term outcomes after their Covid, and children are dying," says Amanda Cohn of the Centers of Disease Control and Prevention, who voted yes. — AFP
New Zealand sets a 90-percent vaccination target Friday for scrapping lockdowns as Prime Minister Jacinda Ardern unveiled a plan to open up despite the stubborn grip of the Delta variant.
Ardern says her goal had shifted from eliminating Covid-19 to minimising its spread in the community by ramping up vaccinations.
She says the change meant New Zealanders would not be subject to stay-at-home orders and business shutdowns, provided they were fully inoculated.
"We cannot ask vaccinated people to stay home forever," she tells reporters. — AFP
An expert committee recommends a booster dose of Moderna's anti-Covid vaccine in the United States for certain at-risk groups, a month after making a similar decision for the Pfizer shot.
The opinion submitted by the advisory committee of the Food and Drug Administration -- composed of researchers, epidemiologists and infectious disease experts -- is not binding, but it is rare for the FDA not to follow it.
After a day of debate, the experts decided to authorize a booster dose of Moderna for three categories of people: the over-65s, people aged between 18 and 64 who are at a higher risk of developing a severe version of the coronavirus, and those whose work may involve frequent exposure to the virus. — AFP
G20 trade ministers on Tuesday promise to work towards a fair distribution of Covid-19 vaccines by lifting export restrictions and making the trade system more transparent.
Their final statement, adopted after a meeting in Sorrento, southern Italy, was a sign of the return of multilateralism, says Italian Foreign Minister Luigi Di Maio.
"We have to ensure that there is greater circulation of vaccines and that there are production factories in the developing countries," French trade minister Franck Riester says. — AFP
COVID vaccinations will be compulsory for all students in California, the state's governor announced Friday — a first in the United States, where vaccine hesitancy has slowed efforts to end the pandemic.
The plan will be phased in as Food and Drug Administration regulators grant full approval for use in younger age groups.
California "will require our kids to get the Covid-19 vaccine to come to school," said Governor Gavin Newsom.
"Our schools already require vaccines for measles, mumps and more. Why? Because vaccines work. This is about keeping our kids safe and healthy."
The Pfizer-BioNTech vaccine has been granted full FDA approval for those age 16 and up. — AFP
France's pharmaceutical giant Sanofi, which has lagged rivals in producing new generation Covid-19 vaccines, said Tuesday it had decided to halt development of an mRNA candidate and focus on another vaccine against coronavirus.
Despite positive results at phase one and two trials, the candidate will not go to the third and final phase, Sanofi said, as they believed it would arrive too late to market with 12 billion anti-Covid doses already due to be produced by the end of the year.
Results from phase three trials of the other vaccine, developed with Britain's GlaxoSmithKline are expected before the end of 2021. — AFP
A committee of US health experts declines to approve Pfizer booster shots for individuals at high risk of COVID-19 exposure due to their jobs, despite authorization from a different agency just the night before.
The decision has contributed to growing confusion about the campaign for booster doses in the United States, which the administration of President Joe Biden announced in mid-August but has since lost momentum.
The Centers for Disease Control and Prevention (CDC) committee voted Thursday to recommend a third dose of Pfizer's vaccine for people over age 65 and for those with underlying conditions who are at risk of developing a severe case of COVID-19. — AFP
The United States authorizes the use of boosters of Pfizer's Covid-19 vaccine for people aged over 65, people at high-risk of severe disease and those in high-exposure settings to the virus.
"Today's action demonstrates that science and the currently available data continue to guide the FDA’s decision-making for COVID-19 vaccines during this pandemic," says Janet Woodcock, acting head of the Food and Drug Administration. — AFP
COVID-19 vaccine manufacturers are putting profit before lives, Amnesty International says, as it demands two billion doses for poorer nations by the end of the year.
The human rights group says in a new report that US President Joe Biden was expected to outline a pledge at the UN General Assembly to fully vaccinate 70% of the world's population by next September.
"We need leaders like President Biden to put billions of doses on the table and deliver the goods, otherwise this is just another empty gesture and lives will continue to be lost," Amnesty chief Agnes Callamard says.
A panel of leading US medical experts advising the government vote in favor of authorizing boosters of Pfizer's coronavirus vaccine for everyone aged 65 and up, as well as people at high risk of developing severe Covid.
The same committee however rejected an initial proposal, submitted by Pfizer and backed by President Joe Biden's administration, to fully approve boosters to everyone aged 16 and over.
The decisions came after a day-long meeting full of data presentations and at times charged debate that was convened by the Food and Drug Administration (FDA). Tens of millions of Americans will soon be eligible for a third shot. — AFP
US medical experts will meet Friday to debate and vote on the controversial question of giving out booster doses of Pfizer's Covid-19 vaccine to the general population.
President Joe Biden's administration announced in August a plan to roll out third shots to everyone, not just the immune compromised already able to receive them, starting from September 20.
But experts have since expressed reservations about whether they are required, amid concerns over global inequity, the greater need to vaccinate the unvaccinated, and possible increased risk of side effects. — AFP
China has fully vaccinated more than one billion people against the coronavirus — 71% of its population — official figures showed Thursday.
The country had mostly curbed the virus within its borders but is racing to get the vast majority of its population vaccinated as a new outbreak takes hold in the southeast.
"As of September 15, 2.16 billion vaccine doses have been administered nationwide," said National Health Commission spokesman Mi Feng at a press briefing.
Chinese health authorities said late last month that 890 million people in China had been fully vaccinated and two billion doses administered.
The government has not publicly announced a target for vaccination coverage, but top virologist Zhong Nanshan said last month that the country is likely to have 80% of its population inoculated by the end of the year.
China is currently battling an outbreak of the Delta variant in the southeastern province of Fujian that has infected almost 200 people so far in three cities, many of whom are schoolchildren. — AFP
Cuba says it would seek World Health Organization approval for two home-grown coronavirus vaccines it hopes to commercialize widely.
A vetting process will start Thursday with WHO experts examining the nation's Abdala and Soberana 02 jabs, says Rolando Perez of state pharma group BioCubaFarma.
Perez says the experts would examine the vaccines' "safety, immunogenicity (the ability of a vaccine to provoke an immune response) and efficacy." — AFP
The European Union is to donate another 200 million COVID-19 vaccine doses to low-income countries, more than doubling its present pledge, the bloc's chief says.
The extra doses announced by European Commission President Ursula von der Leyen come on top of 250 million shots the EU has already promised to give to other countries, particularly ones in Africa.
"I can announce today that the commission will add a new donation of another 200 million doses until the middle of next year," she tells the European Parliament in her annual State of the European Union address. — AFP
The EU is to donate another 200 million Covid-19 vaccine doses to low-income countries, more than doubling its present pledge, the bloc's chief Ursula von der Leyen says on Wednesday.
"I can announce today that the (European) Commission will add a new donation of another 200 million doses until the middle of next year. This is an investment in solidarity, and it is an investment also in global health," she tells the European Parliament in her annual State of the European Union address. — AFP
Africa wants to buy COVID-19 vaccines, rather than keep waiting for donor-funded doses to arrive, the African Union says, imploring producers to give the continent a fair shot at market access.
The AU also urged manufacturing nations to lift export bans so the continent can begin to address for itself the glaring inequity in access to coronavirus jabs, as wealthy nations hog available doses.
"Vaccine sharing is good. But we shouldn't have to be relying on vaccine sharing," Strive Masiyiwa, the AU's COVID-19 special envoy, tells a press conference at the World Health Organization in Geneva.
Zimbabwe's parliament on Tuesday bans anyone not vaccinated against Covid-19 from attending church services, the latest in a series of measure to boost uptake of the coronavirus jab.
The southern African country had already made the vaccine mandatory for civil servants and teachers earlier this month.
Getting vaccinated is also a prerequisite for trading in markets, working out at gyms, frequenting restaurants and sitting university exams.
"With regards to churches, Cabinet has resolved that only vaccinated congregants can attend," says a statement issued after the cabinet meeting. — AFP
Britain will send four million Covid-19 vaccine doses to Australia, Prime Minister Scott Morrison announced Friday, as his country raced to halt a deadly virus outbreak.
The Australian leader said the planes delivering Pfizer vaccines were "on the tarmac" in the UK and would deliver "four million doses of hope" within weeks.
A first batch 292,000 doses will be shipped "shortly", the British government said in a separate statement. — AFP
The European Union and UK-based drugs giant AstraZeneca announce Friday that they had reached a settlement in a dispute over a shortfall in coronavirus vaccine deliveries.
The agreement will see the firm deliver the rest of the 300 million doses it promised under contracts with the EU before the end of March 2022, and brings to an end a battle in the Belgian courts.
Brussels was furious when the British-Swedish pharmaceutical outfit fell far short of its delivery promises, undermining the early stages of the EU COVID-19 vaccine rollout. — AFP
Britain will send four million COVID-19 vaccine doses to Australia, Prime Minister Scott Morrison announces, as his country raced to halt a deadly virus outbreak.
The Australian leader says the planes delivering Pfizer vaccine doses were "on the tarmac" in the UK and would deliver "four million doses of hope" within weeks.
Several of Australia's largest cities are in lockdown and case numbers and deaths are steadily rising, as the highly transmissible Delta variant spreads throughout the country. — AFP
Taiwan receives its first batch of Pfizer-BioNTech coronavirus vaccines, a delivery organized by two tech giants and a charity because of diplomatic pressure from China.
The 930,000 doses are the first of 15 million jabs acquired by Foxconn and Taiwan Semiconductor Manufacturing Company (TSMC), as well as Buddhist charity Tzu Chi foundation, in deals with a China-based distributor after months of wrangling.
Despite donations of several million doses from the United States and Japan in June, Taiwan has been struggling to secure enough vaccines for its 23.5 million population and its precarious political status has been a major stumbling block.
The United States has thrown away at least 15.1 million doses of Covid-19 vaccines since March 1, according to a report by NBC News.
The figure is far higher than previously thought and probably still an undercount, because it is based on self-reported data from pharmacies, states and other providers, NBC says, quoting a response it received to a request for public data.
At least seven states are missing from the figures, as well as major federal agencies. — AFP
EU chief Ursula von der Leyen said Tuesday 70% of adults in the European Union were now fully vaccinated against COVID-19, hitting an end-of-summer target the bloc set for itself in January.
"Today we reached an important milestone in our vaccination campaign. Seventy percent of adults in the EU are now fully vaccinated and that means 250 million people are fully immunized," Von der Leyen said in a video posted online.
Von der Leyen heads the European Commission, which is responsible for ordering vaccines for the EU's 27 member states, and had already announced in July that 70% of the adult population of the EU had received at least one dose.
The global fight against the coronavirus pandemic is now dominated by the battle against the Delta variant, a more contagious version of the COVID-19 coronavirus.
The World Health Organization fears that the pandemic could kill an additional 236,000 people in Europe by December 1 and has expressed concern about the stagnating pace of vaccinations on the continent. — AFP
Teachers and school staff should be among the groups prioritised for Covid-19 vaccinations so that schools in Europe and Central Asia can stay open, the World Health Organization (WHO) and Unicef said on Monday.
"This is of paramount importance for children's education, mental health and social skills, for schools to help equip our children to be happy and productive members of society," the director of the WHO European region, Hans Kluge, said in the statement.
"The pandemic has caused the most catastrophic disruption to education in history," he added. — AFP
India has given more than 10 million Covid-19 jabs in a single day for the first time, authorities said Saturday, as the South Asian giant bolsters its defences for a predicted new surge.
The health ministry said the 10 million landmark was passed on Friday, beating the country's previous daily record of 9.2 million. The government has been stung by criticism after a brutal coronavirus wave in April and May killed more than 200,000 people.
Prime Minister Narendra Modi hailed the milestone as a "momentous feat" for the nation of 1.3 billion people. — AFP
Japan's Okinawa region suspended the use of Moderna's COVID-19 vaccine on Sunday after another contamination was spotted, the local government said.
It comes a day after the Japanese health ministry said it was investigating the death of two men who received shots from tainted Moderna batches — though the cause of their death is unknown.
The Okinawa prefecture, in southern Japan, said Sunday's vaccination programme was partially postponed.
"We are suspending the use of Moderna's Covid-19 vaccines as foreign substances were spotted in some of them," it said in a statement. — AFP
Authorities say that India has given more than 10 million COVID-19 jabs in a single day for the first time as the South Asian giant bolsters its defenses for a predicted new surge.
The health ministry says the 10 million landmark was passed on Friday, beating the country's previous daily record of 9.2 million. The government has been stung by criticism after a brutal coronavirus wave in April and May killed more than 200,000 people.
Prime Minister Narendra Modi hailed the milestone as a "momentous feat" for the nation of 1.3 billion people. — AFP
Japan will halt the use of 1.63 million doses of Moderna's COVID-19 vaccine after reports of contamination in several vials, drugmaker Takeda and the health ministry say.
Takeda, which is in charge of sales and distribution of the Moderna shot in Japan, said it had "received reports from several vaccination centres that foreign substances have been found inside unopened vials."
"Upon consultation with the health ministry, we have decided to suspend the use of the vaccine" from three whole batches from Thursday, it adds. — AFP
Japan will halt the use of 1.63 million doses of Moderna's Covid vaccine after reports of contamination in several lots, drugmaker Takeda and the health ministry says Thursday.
Takeda, which is in charge of sales and distribution of the Moderna shot in Japan, says it had "received reports from several vaccination centres that foreign substances have been found inside unopened vials from specific lots."
"Upon consultation with the health ministry, we have decided to suspend the use of the vaccine from the lot from August 26," it adds. — AFP
The medication regulatory body says Cuba approved two more domestic COVID-19 vaccines for emergency use.
The authorization came after a "rigorous evaluation process of the Soberana 02 and Soberana Plus vaccines," the body, CECMED, says in a statement.
The two Soberana vaccines are complementary. — AFP
A "no jab, no job" coronavirus policy went into effect in Fiji on Sunday, with unvaccinated public servants forced to go on leave as the Pacific nation joined a number of countries in imposing similar mandates.
A stubborn outbreak of the highly infectious Delta variant that started in April ended a yearlong spell of no community transmission of COVID-19, and has overwhelmed Fiji's healthcare system with more than 40,000 cases.
The government has argued that mandatory vaccinations are necessary to raise immunisation rates and end the outbreak.
After a period of forced leave starting Sunday, Fiji's public servants who remain unvaccinated by November will be dismissed. — AFP
Canadians wanting to eat at a restaurant, go to a bar or gym, or attend a festival in Quebec will have to present a vaccine passport starting September 1, officials announce Tuesday.
The province will be the first in Canada to require such passes, which are increasingly being used across the world to limit entry to public places to those who have been vaccinated, recovered from Covid-19 or tested negative.
They are also hugely controversial in some jurisdictions leading to mass protests against mandatory inoculations.
"Our objective with the passport is not to go backwards to a lockdown and, at the same time, to avoid overloading our hospitals," Quebec Health Minister Christian Dube tells a news conference. — AFP
The first generation vaccine developed by BioNTech-Pfizer works against coronavirus variants such as the Delta strain and does not need to be modified for the moment, the chief executive of German company BioNTech said Monday.
"It is quite possible that in the next six to 12 months, further variants will emerge and that would require adaptation of the vaccine but it is at the moment not yet the case," Ugur Sahin told journalists. — AFP
Cambodia began a roll out of COVID-19 vaccinations for teenagers in its capital Phnom Penh and three provinces Sunday, with Premier Hun Sen's grandchildren among the first to get the jab.
Several countries in Southeast Asia are currently experiencing a deadly COVI-19 resurgence largely driven by the highly contagious Delta variant, which has set back the region's successes last year in curbing pandemic tolls.
While Cambodia appeared to have escaped the brunt of the virus last year, an outbreak first detected in February has steadily driven up the caseload to nearly 78,000.
On Sunday, the kingdom began vaccinating children between the ages of 12 to 17, starting the campaign in its capital Phnom Penh, as well as in the three hardest-hit provinces of Kandal, Koh Kong and Preah Sihanouk.
"The vaccination for children today is a key step to herd immunity in communities," said the Cambodian leader. — AFP
The US Department of Veterans Affairs said Monday its more than 100,000 health care personnel would have to get COVID-19 shots, becoming the first federal agency to institute a mandate as California and New York City announced new vaccine requirements affecting workers.
US President Joe Biden's administration had previously been reluctant to support such measures, but is now confronted with a Delta variant-driven surge that is sweeping mainly through America's tens of millions of unvaccinated.
"Whenever a Veteran or VA employee sets foot in a VA facility, they deserve to know that we have done everything in our power to protect them from COVID-19," said VA Secretary Denis McDonough in a news release. — AFP
Jordan's health ministry announces Saturday that Covid-19 vaccines will now be available for children aged 12 and above.
The ministry "has decided to lower the Covid-19 vaccination age to 12 years, starting from Sunday July 25" and without requiring an appointment, the ministry says in a statement on its Facebook page on Saturday.
"Vaccination will be optional, and those under 18 will be able to receive the Pfizer-BioNTech vaccine with the consent of their guardian," the statement adds. — AFP
Covid-19 vaccine makers BioNTech and Pfizer say they had found a South African partner to produce their jab locally, the first such deal on the African continent.
The move comes amid growing criticism of vaccine inequality that has seen poor countries fall behind richer ones in the race to protect people from the coronavirus.
Under the agreement, Cape Town-based Biovac will complete the last step in the manufacturing process of the Pfizer/BioNTech vaccine, known as "fill and finish", the companies said in a statement. — AFP
Half a million Covid-19 vaccines began shipping Thursday from the United States to Jordan in the latest stage of a global US effort to overcome the pandemic, officials said.
A senior White House official told AFP: "500,000 doses of Pfizer will begin to be shipped to Jordan from the United States. These doses arrive in Jordan on Saturday."
According to the official, who spoke on condition of anonymity, the doses are being sent in a direct arrangement with Jordan, rather than through the international Covax program. — AFP
Thailand defends mixing two different COVID-19 vaccines to battle a surge in infections, after the WHO's top scientist warned it was a "dangerous trend" not backed by evidence.
The kingdom is struggling to contain its latest outbreak fuelled by the highly contagious Delta variant, with cases and deaths skyrocketing and the healthcare system stretched thin.
Authorities say they will mix a first dose of the Chinese-made Sinovac jab with a second dose of AstraZeneca to try and achieve a "booster" effect in six weeks instead of 12. — AFP
Cuba approves its home-grown Abdala vaccine for emergency use, the first Latin American coronavirus jab to reach this stage and a possible lifeline for a region trying to battle a killer pandemic with modest means.
The country's CECMED health regulator gives the go-ahead after Abdala's makers last month announced the vaccine candidate was more than 92% effective at preventing COVID-19 disease after three doses.
Cuba is working on five coronavirus vaccines, and in May started immunizing its population using two of them -- Abdala and Soberana 2 -- even before they received approval. — AFP
The United States sends Vietnam two million doses of Covid-19 vaccine Tuesday, the White House says, in its latest assistance to countries struggling to tame the pandemic.
The Moderna vaccine shipment — part of a first 80 million doses that President Joe Biden has pledged to allocate worldwide — should arrive in Vietnam this weekend, a White House official, who spoke on condition of anonymity, tells AFP.
"This is just the beginning of doses being shipped to southeast Asia," the official says. — AFP
President Vladimir Putin says Wednesday he opposes introducing mandatory vaccinations in Russia despite a surge in coronavirus infections in the country and sluggish inoculation rates.
"I do not support mandatory vaccinations," Putin tells Russians during his annual phone-in broadcast on television.
Asked if he supported a new nationwide lockdown, he says regional authorities were instead promoting localised mandatory vaccinations and other measures to avoid introducing new quarantines. — AFP
Neither the threat of dying from Covid nor an array of inducements from lottery tickets to guns and marijuana have been enough to sway America's staunchest vaccine holdouts.
As the divide between the country's pro- and anti-immunization regions widens, and the dangerous Delta variant keeps gaining ground, experts are calling for more mandates in jurisdictions, colleges and businesses so Americans are protected where they live and work.
The idea has sparked controversy in a nation that cherishes individual liberty, but these concerns need to be weighed against collective wellbeing, Gregory Poland, a professor of medicine and infectious diseases at Mayo Clinic in Minnesota told AFP.
"What happens when the world's on fire? Do you allow people to stand there with matches and gasoline? What happens with the disease where the decision you make not only affects you, but affects people around you, or that you've come into contact with?" he said. ?— AFP
India on Tuesday authorises the emergency use of Moderna's Covid-19 vaccine as it seeks to ramp up its vaccination drive in the wake of a record-breaking surge in infections and deaths.
Moderna's shot is the fourth to be approved by New Delhi after Oxford-AstraZeneca's Covishield and Covaxin, which was developed by Indian firm Bharat Biotech, and Russia's Sputnik V.
"I am pleased to inform that an application received from Moderna through an Indian partner of theirs, Cipla, has been granted EUA (Emergency Use Authorisation)," a member of government advisory body NITI Aayog, Vinod K. Paul, says at a health ministry briefing. — AFP
French pharmaceutical giant Sanofi says Tuesday that it would invest 2 billion euros ($2.4 billion) in the mRNA vaccine technology behind the pioneering Covid-19 jabs developed by rivals BioNTech-Pfizer and Moderna.
It says it would set up an mRNA "centre of excellence" employing 400 people at its laboratories in the US city of Cambridge and Marcy-L'Etoile near the French city of Lyon.
"This massive new investment clearly puts us in the race to develop next-generation vaccines where mRNA technologies can have greatest impact," Thomas Triomphe, global head of Sanofi Pasteur, says in a statement. — AFP
A panel of experts convened by the top US health agency will hold a meeting Wednesday to review data surrounding more than 300 confirmed cases of heart muscle inflammation among adolescents and young adults after receiving mRNA Covid vaccines.
The committee, hosted by the Centers for Disease Control and Prevention (CDC), will hear a risk-benefit analysis as researchers explore whether the shots can cause myocarditis, as well as cases of inflammation of the heart lining, pericarditis.
Israel first was the first country to identify a possible link.
"These cases are rare, and the vast majority have fully resolved with rest and supportive care," said CDC director Rochelle Walensky last week ahead of the meeting, which was initially scheduled for last Friday but postponed because of a new public holiday. — AFP
Cuba's Abdala coronavirus candidate vaccine showed "efficacy" of more than 92% after three doses, its maker says Monday, though it did not specify whether this was measured against infection, disease, or death.
Cuba is working on five coronavirus vaccines, and last month started immunizing its population using two of them yet to complete clinical trials. — AFP
China has now administered more than a billion doses of Covid vaccines, the country's health authority said Sunday, more than a third of the number given worldwide.
It comes after the number of doses administered globally surpassed 2.5 billion on Friday, according to an AFP count from official sources.
— AFP
The Palestinian Authority says it cancelled a swap deal that would have seen Israel provide it with one million COVID-19 jabs, as the doses were "about to expire".
Israeli officials earlier Friday had announced the deal, saying the Jewish state was to provide the doses to the Palestinian Authority as their expiry date loomed.
The PA, based in the occupied West Bank, had confirmed the delivery "in the coming days" of a million vaccine doses, without mentioning an agreement with the Jewish state. — AFP
Australia recommends that AstraZeneca's Covid-19 jab should not be given to people under 60 on Thursday, a fresh blow to the country's glacial vaccine rollout.
Health Minister Greg Hunt says concerns over possible links to blood clots meant Pfizer was now "the preferred vaccine" for everyone under 60 years old.
Australian authorities had already restricted the AstraZeneca shot to those over 50 in April, after several cases of severe blood clots were possibly linked to it. — AFP
Covid vaccine-maker AstraZeneca reveals it had hit a setback in trials of a treatment for the coronavirus.
The drug, made from a combination of two antibodies, failed its main goal to treat COVID-19 symptoms in exposed patients, AstraZeneca says in a statement.
The treatment has been undergoing phase 3 or final clinical trials to assess its safety and efficacy. — AFP
The US Food and Drug Administration says it had told Johnson & Johnson that millions of doses of COVID-19 vaccine produced at a troubled plant can't be used because of possible contamination issues.
In a statement, the FDA says "several" batches of vaccine manufactured at the Emergent BioSolutions facility in the city of Baltimore are not suitable for use. Each batch is known to correspond to several million doses.
Neither the agency nor J&J revealed the precise number of doses, but The New York Times placed the number at 60 million, quoting people familiar with the matter. — AFP
G7 leaders meet for their first in-person talks in nearly two years, with an expected pledge to donate one billion COVID-19 vaccine doses to the world's poorest countries, as part of a show of Western democratic unity against the planet's most pressing issues.
The club of leading economies -- Canada, France, Germany, Italy, Japan, the UK and United States -- say a joint approach is the world's best chance for recovering from the global health crisis, and tackling climate change.
President Joe Biden set the tone on Wednesday, ditching Donald Trump's isolationist stance on global affairs to ram home a message of resolve by the G7 and NATO against both Beijing and Moscow as he heads on to his first sit-down with Russian President Vladimir Putin next week in Geneva. — AFP
G7 leaders open a three-day summit aimed at helping to end the COVID-19 pandemic and forge a climate-centric economic recovery, after pledging to donate one billion vaccine doses for the world's poorest countries.
US President Joe Biden and his colleagues from Britain, Canada, France, Germany, Italy and Japan will sit down for their first face-to-face gathering in nearly two years, after the pandemic wiped out last year's summit.
Meeting under the protection of a smothering security operation in the Cornish resort of Carbis Bay, southwest England, the leaders are also expected to address warnings to Russia and China. — AFP
Lebanon's cash-strapped leaders are bribing their base with free COVID-19 jabs ahead of next year's elections, in what observers say is the latest variant on an old corruption trick.
The "vaccine for vote" system builds on decades-old patronage practices that have seen leaders buy their way into office by offering voters money or public sector employment.
But with state resources stretched to their limit by a severe economic crisis and international aid dwindling due to a failure to deliver promised reforms, politicians are turning to COVID jabs to stock up on political capital.
"Political forces are trying to directly or indirectly make themselves a part of the equation with regards to the vaccine campaign, primarily because it is a profitable investment," said a member of the state-run National Vaccination Committee who spoke on condition of anonymity. ?— AFP
Sri Lanka began injecting pregnant women with a Chinese coronavirus vaccine on Wednesday and Nepal resumed inoculations with a China-made jab as India's neighbours turn to Beijing and Moscow for help with supplies.
Nepal halted inoculations at the end of May after its stock of AstraZeneca shots and Chinese Sinopharm jabs ran short.
The programme resumed on Tuesday after a million more Sinopharm doses arrived from China, the only country that has so far responded to its appeals for help.
India had previously supplied Nepal with the AstraZeneca vaccine from its manufacturer Serum Institute but in March froze vaccine exports as infections soared domestically.
"Nepal has sent requests to many countries including both neighbours, US, Russia and other countries but no additional vaccine has arrived yet," health ministry official Samir Kumar Adhikari told AFP. — AFP
China has approved the emergency use of a COVID-19 vaccine for those as young as three, the drugmaker confirms Tuesday, making it the first country to offer jabs to young children.
Since the coronavirus first emerged in central China, Beijing has mostly managed to bring the country's outbreak under control, and has administered over 777 million vaccine doses after a sluggish start.
A spokesperson for Sinovac told AFP its vaccine had been approved for use on children. — AFP
US biotech firm Moderna says Monday it was seeking conditional approval for use of its Covid-19 vaccine on teens in the European Union and Canada, in a boost for inoculation campaigns as the summer begins.
The firm says it also plans to file for emergency approval with the Food and Drug Administration in the United States, where the Pfizer/BioNTech jab is already being administered to adolescents age 12 and up.
"We are pleased to announce that we have submitted for conditional marketing approval of our Covid-19 vaccine with the European Medicines Agency for use in adolescents in the European Union," Moderna CEO Stephane Bancel says. — AFP
Thailand launches a mass Covid-19 vaccination drive on Monday as it seeks to beat a third wave of infections and reboot its vital tourism industry, which has been devastated by pandemic travel curbs.
The kingdom plans to give out some six million jabs in June, initially focusing on Bangkok — where the country's current third wave began — and the tourist island of Phuket.
The push comes as the government faces criticism over the slow pace of its vaccination strategy — less than three million of the 70 million population have got a shot — and concerns over supplies. — AFP
Popular dating apps on Monday launched a campaign encouraging British users to post "I got my shot" on their profiles as the UK rollout reaches young adults.
In partnership with the government, apps including Tinder, Bumble and Hinge are offering special stickers, badges and bonuses for users who say they have had the coronavirus jab, the Department of Health said.
This comes as the UK rollout this week reaches the under-30s, as Health Secretary Matt Hancock confirmed Sunday.
Vaccines minister Nadhim Zahawi said he was "thrilled that we are partnering up with dating apps to boost vaccine uptake across the country".
The United States launched a similar initiative in May, linking up with apps including Tinder, OKCupid and Plenty of Fish. — AFP
Ministers of the APEC trade group on Saturday agreed to improve efforts to expedite the trade of Covid-19 vaccines and medical material across the region.
The June 4-5 meeting of the trade officials, chaired by New Zealand Minister for Trade and Export Growth Damien O'Connor, was held online due to the pandemic.
In a joint statement, the Asia-Pacific Economic Cooperation (APEC) trade ministers said they agreed on "the essential role of trade in tackling the impacts of the COVID-19 pandemic and in enabling a strong economic recovery." — AFP
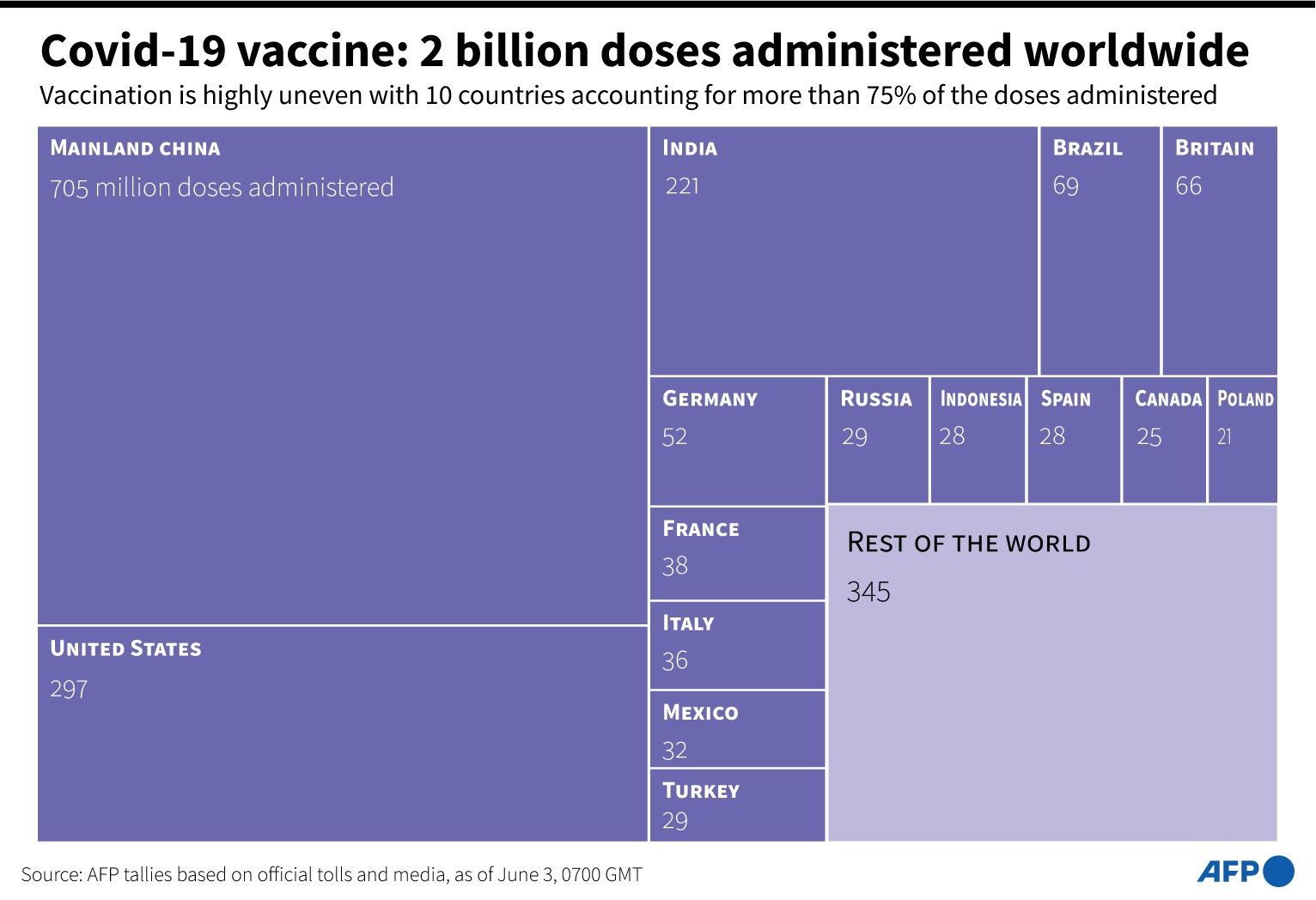
More than two billion COVID-19 vaccines have been given across the world, according to an AFP tally from official sources.
The milestone comes six months after the first vaccination campaigns against COVID-19 began.
At least 2,109,696,022 shots have been given in 215 countries and territories, according to the count from an AFP database taken at 0915 GMT. — AFP
Britain's medicines regulator says the Pfizer/BioNTech vaccine is safe for adolescents aged 12 to 15 after a "rigorous review", following similar assessments in the European Union and United States.
The Medicines and Healthcare products Regulatory Agency (MHRA) approved the two-shot jab following clinical trials among younger age groups, saying the vaccine had met the "expected standards" of safety, quality and effectiveness.
A government committee on vaccination will now decide if and when to begin administering doses to the age bracket. So far it has promptly followed all MHRA rulings on vaccines offered by Pfizer and other companies. — AFP
Tokyo is donating more than one million doses of the AstraZeneca coronavirus vaccines to Taiwan, Japan's foreign minister announces, as Taipei struggles to secure jabs, accusing China of interference.
The move is likely to stir controversy with Beijing, which views democratic and self-ruled Taiwan as its own territory and works to keep the island diplomatically isolated.
"We have received requests from various countries and areas for the provision of vaccines," Toshimitsu Motegi tells reporters in Tokyo. — AFP
More than two billion Covid-19 vaccines have been given across the world, according to an AFP tally Thursday drawn from official sources.
The milestone comes six months after the first vaccination campaigns against Covid-19 began.
At least 2,109,696,022 shots have been given in 215 countries and territories, according to the count from an AFP database taken at 0915 GMT. — AFP
Canadian health authorities announce they are pushing back the expiration date on nearly 50,000 doses of AstraZeneca coronavirus vaccine by one month.
Health Canada says in a statement its approval to extend the shelf life of two lots of vaccine from May 31 to July 1 was supported by "scientific evidence."
"This change will ensure that provinces and territories are able to use up their existing inventory and provide Canadians access to much needed doses of the vaccine," the agency says. — AFP
Rwandan President Paul Kagame describes vaccine distribution in Africa as "scandalously inefficient" and warns against building an "invisible wall" around parts of the world unable to secure jabs.
The World Health Organization (WHO) in May said two percent of COVID-19 vaccines globally had been administered in Africa, a continent of over 1.2 billion people.
Kagame says efforts to ensure fair vaccine access, including the WHO-backed Covax initiative, remained "scandalously inefficient" and added that depriving Africa risked prolonging the pandemic. — AFP
The Covax global vaccine-sharing programme says it needs $2.0 billion in additional funding by the beginning of June in order to boost coronavirus inoculation programmes in lower-income countries.
"We need an additional $2 billion to lift coverage... up to nearly 30 percent, and we need it by June 2 to lock in supplies now so that doses can be delivered through 2021, and into early 2022," the mechanism's organizers -- which include the World Health Organization and the Gavi alliance -- says in a statement.
"If the world's leaders rally together, the original Covax objectives -– delivery of two billion doses of vaccines worldwide in 2021, and 1.8 billion doses to 92 lower-income economies by early 2022 -- are still well within reach," the statement says. — AFP
People in Chile who have been vaccinated and are fully immunized against the coronavirus could from Wednesday obtain a pass to move about more freely, a move criticized by health professionals.
The pass, which can be downloaded on a person's mobile phone two weeks after receiving their second shot, gives the bearer permission to move about freely in cities under lockdown to go to the supermarket, pharmacy, or for open air exercise.
Those without permits, by comparison, have to print out a permission form every time they want to leave the house, which is allowed for essential reasons only.
Pass holders can also travel between cities and towns not under lockdown.
Face masks remain compulsory for everyone, indoors and out, and the pass will not be valid during the daily 10.00 pm-to-5.00 am curfew. It also does not replace the permission people need to go back to work, the government has said. — AFP
The European Commission will confront drugs giant AstraZeneca in a Belgian court on Wednesday over coronavirus vaccine delivery shortfalls that hampered efforts to kickstart inoculations across the bloc.
Lawyers for both sides are due to appear before a judge in the French-speaking court in Brussels from 09:00 am (0700 GMT). Another hearing is scheduled for Friday, the court said.
The EU is suing the British-Swedish pharmaceutical group in a bid to force it to deliver 90 million more doses of its Covid-19 vaccine before July.
The deadline for the contract was set for mid-June, according to the Commission, and the EU says the company will face financial penalties if it does not meet this deadline.
AstraZeneca delivered only 30 million doses in the first quarter out of the 120 million it was contracted to supply. For the current quarter which runs until June 30, it plans to deliver only 70 million of the 180 million initially promised.
A Commission official close to the case told AFP this month that AstraZeneca was currently delivering doses at a rate of only 10 million per month, well below the planned pace.
The group denies having failed in its obligations and at the end of April denounced the lawsuit as "unfounded".
One lawyer for AstraZeneca claimed that the EU had been warned "as early as February" of the delays and expressed surprise that the bloc had waited at least two months to take the matter to court.
The EU is also accusing the pharmaceutical giant -- which worked with Oxford University in the development of its vaccine — of having favoured the UK in its deliveries, even for jabs made by subcontractors on the continent.
AstraZeneca's French-Australian boss Pascal Soriot has argued that his company's contract with the EU binds it only to a "best reasonable efforts" clause.
But the European Commission says the rest of the contract shows greater legal responsibility than that, and EU diplomats and lawmakers have pointed out that the company has largely delivered promised doses to Britain, where it is headquartered. — AFP
US biotech firm Moderna says Tuesday that trials had shown its Covid-19 vaccine is "highly effective" in adolescents aged 12-17 and the company would seek regulators' approval in June.
"We are encouraged that mRNA-1273 was highly effective at preventing Covid-19 in adolescents," CEO Stephane Bancel says in a statement.
"We will submit these results to the US FDA and regulators globally in early June and request authorization." — AFP
Denmark plans to donate three million COVID-19 doses to developing countries this year through the Covax global sharing scheme, Prime Minister Mette Frederiksen says.
Frederiksen makes the announcement on her arrival in Brussels for an EU summit.
"We have purchased quite a few vaccines, so we have scope to vaccinate the Danish people, revaccinate in the autumn if necessary and donate vaccines," Danish news agency Ritzau quotes Frederiksen as saying. — AFP
Italy passes the milestone of 30 million doses injected in its Covid-19 vaccination effort, with nearly 10 million people in the country now fully vaccinated.
More than 9.85 million people in Italy have received two doses of a coronavirus vaccine, according to government figures, representing 16.6 percent of the 60 million-strong population.
Two thirds of the doses administered so far in Italy have gone to those aged over 60. — AFP
Japan formally approves Moderna and AstraZeneca's COVID-19 vaccines, but the latter will not be used immediately because of lingering concern over very rare blood clots.
The decision comes just over two months before the pandemic-postponed Olympics, with growing disquiet in Japan about the country's comparatively slow vaccine rollout.
Nine regions including Tokyo are already under a virus state of emergency, with the measure now being expanded to Okinawa in the south. — AFP
The UN Security Council issues a unanimous declaration underlining the need to increase COVID-19 assistance to Africa, in particular by boosting vaccine supplies.
"The Security Council expresses concern that Africa has only received two percent of all vaccines administered globally," says the text, which was adopted during a virtual session on peace and security in Africa organized by China.
The Council stresses "the need to enable equitable access to quality, safe, efficacious, and affordable COVID-19 diagnostics, therapeutics, medicines and vaccines." — AFP
The Pfizer-BioNTech Covid-19 vaccine can be stored at refrigerator temperatures for up to a month in the United States, the country's health regulator announces, in a change expected to help distribution of the shot.
The US Food and Drug Administration said it had made the decision "based on a review of recent data submitted by Pfizer," and will allow vials of the vaccine to be stored at refrigerator temperatures of 2-8 degrees Celsius (35-46 degrees Fahrenheit) for up to one month.
The vials were previously only allowed to be kept at such temperatures for five days. — AFP
British Prime Minister Boris Johnson says that COVID-19 vaccines are proving effective against a highly contagious variant from India as he was pressed on why the government is allowing travel from hotspots.
Johnson has been under particular pressure for delaying restrictions on travel to and from India last month, at a time when he was still planning to head to a trade-focused summit in New Delhi.
The trip was eventually called off as India succumbed to a devastating new wave of infections, and it was then quickly added to the UK's "red list", meaning arriving travellers have to quarantine in hotels at their own cost. — AFP
Countries belonging to the G7 and the European Union can afford to donate more than 150 million vaccines to countries in need without compromising their own goals, UNICEF said Monday.
The world's seven richest states and the EU could help close the world's vaccine gap by sharing just 20 percent of their June, July and August stocks with the Covax jab scheme for poorer nations, a study by British firm Airfinity showed.
"And they could do this while still fulfilling their vaccination commitments to their own populations," UNICEF director Henrietta Fore said.
The UK is due to host its fellow G7 member states Canada, France, Germany, Italy, Japan and the US for a summit in June.
By that time UNICEF said the Covax program being co-led by Gavi the Vaccine Alliance, along with the World Health Organization (WHO) and Coalition for Epidemic Preparedness Innovations (CEPI) will find itself 190 million doses short of what it had planned to distribute. — AFP
Britain is confident that existing vaccines will provide protection from a more transmissible Indian coronavirus variant now spreading across the country, Heath Secretary Matt Hancock says.
And hours later, he was able to announce that the country had passed the milestone of 20 million adults vaccinated with two doses of the coronavirus jab.
The news came just before England, Scotland and Wales are set to unlock parts of their economy on Monday. — AFP
France is on track to reach its goal Saturday of 20 million initial doses of coronavirus vaccines, officials say days ahead of a hugely anticipated reopening of restaurant terraces as the government begins lifting a nationwide lockdown.
"Again nearly 600,000 vaccinations today," Health Minister Olivier Veran tweeted late Friday. "Tomorrow, 20 million French will have had at least one dose," which would represent nearly 30% of the population. — AFP
Sao Paulo, Rio de Janeiro and other Brazilian states suspend immunization of pregnant women with the AstraZeneca coronavirus vaccine on the advice of the national health regulator after a reported death.
The suspension was to remain in place until the health ministry establishes the cause of death of a woman in Rio de Janeiro who received the vaccine, Health Minister Marcelo Queiroga tells a news conference.
Queiroga says the call was made by a team of experts and was based "exclusively on technical criteria." — AFP
Brazil will begin vaccinating Olympic athletes and other members of its Tokyo-bound delegation against COVID-19 this week, officials said Tuesday.
The hard-hit South American country is struggling to secure enough vaccines for its 212 million people, but will be able to immunize its Olympians thanks to donated doses from pharmaceutical companies, health officials said.
Health Minister Marcelo Queiroga told a news conference that 1,814 members of the delegation would be vaccinated starting Wednesday, including Olympic and Paralympic athletes, coaches, staff members and journalists traveling to the Games.
"The doses will be donated by pharmaceutical companies so that (the program) will not affect our national immunization campaign," he said.
Pfizer is expected to donate 4,050 doses of its vaccine and Chinese lab Sinovac 8,000, officials said. — AFP
The US Food and Drug Administration (FDA) on Monday authorized the use of the Pfizer-BioNTech Covid-19 vaccine for children aged 12 to 15 years old.
"This is a promising development in our fight against the virus," said President Joe Biden.
"If you are a parent who wants to protect your child, or a teenager who is interested in getting vaccinated, today's decision is a step closer to that goal."
The FDA previously granted an emergency use authorization for the Pfizer-BioNTech vaccine to individuals aged 16 and older. — AFP
German firm BioNTech says Monday that the Covid-19 vaccine it developed with Pfizer does not require any modifications at the moment to protect against variants of the virus.
"To date, there is no evidence that an adaptation of BioNTech's current Covid-19 vaccine against key identified emerging variants is necessary," the company says in a statement.
Nevertheless, in preparations for a need at some point to make tweaks to its current vaccine, the company says it began tests in March on a "modified, variant-specific version" of its jabs. — AFP
The EU has concluded a deal with BioNTech/Pfizer for up to 1.8 billion extra doses of its COVID-19 vaccine, European Commission chief Ursula von der Leyen says on Saturday.
"Happy to announce that the EU Commission has just approved a contract for guaranteed 900 million doses (+900 million options) with BioNTech/Pfizer for 2021-2023," she tweets from an EU summit in Portugal.
"Other contracts and other vaccine technologies will follow," she promises. — AFP
Pope Francis offers his support Saturday for waiving coronavirus vaccine patents to boost supply to poorer countries, in a video message to the "Vax Live" concert.
The Argentine pontiff backs "universal access to the vaccine and the temporary suspension of intellectual property rights" in the recording made in his native Spanish.
Francis, who has repeatedly spoken of the need to share vaccines, condemns the "virus of individualism" that "makes us indifferent to the suffering of others." — AFP
Russian President Vladimir Putin on Thursday said he supported the idea of a waiver on patent protections for coronavirus vaccines and urged his government to consider lifting them for Russia's jabs.
A campaign to lift patent protections on Covid-19 vaccines picked up steam on Thursday, with French, German and EU leadership saying they were ready to discuss a proposal by US President Joe Biden before Putin added his voice in support.
"We are hearing from Europe an idea that, in my opinion, deserves attention -- namely, to remove patent protections from vaccines against COVID-19 altogether," Putin said during a televised meeting with Deputy Prime Minister Tatiana Golikova.
"Russia would of course support such an approach," Putin said, urging Golikova to work out the logistics.
"As I have said many times... We should not think about how to extract maximum profit, but about how to ensure people's safety." — AFP
Germany is ready to hold talks on a US-backed proposal to waive patents on Covid-19 vaccines, Foreign Minister Heiko Maas says.
"It is a discussion that we're open to," Mass tells reporters when asked about US Trade Representative Katherine Tai's announcement Wednesday that Washington now supports calls for a global waiver on patent protections for Covid-19 vaccines while the pandemic rages.
Maas says Germany would join the discussion about such a move given the "extraordinary situation" of the global coronavirus outbreak. — AFP
The International Federation of Pharmaceutical Manufacturers and Associations expresses disappointment Wednesday at the United States' decision to support a global waiver on patent protections for Covid-19 vaccines.
"As we have consistently stated, a waiver is the simple but the wrong answer to what is a complex problem," the lobby group says in a statement, describing the US move as "disappointing". — AFP
Britain is spending £29.3 million ($40.6 million, 33.8 million euros) on new coronavirus vaccine laboratories in an effort to "future-proof the country from the threat of new variants", the government said Wednesday.
The new labs at the Porton Down research facility in southwest England will be used to test the effectiveness of vaccines against variants and speed up their deployment, according to Health Secretary Matt Hancock.
"We've backed UK science from the very start of this pandemic and this multi-million pound funding for a state-of-the-art vaccine testing facility at Porton Down will enable us to further future-proof the country from the threat of new variants," he says.
The Group of Seven wealthy democracies will discuss coronavirus vaccines Wednesday as they face growing pressure to share stockpiles and know-how with poor nations trailing far behind on fighting the pandemic.
Foreign ministers of Britain, Canada, France, Germany, Italy, Japan and the United States are wrapping up three days of talks in central London that will set the agenda for a G7 leaders' summit next month in Cornwall, southern England.
After a day focused on showing a common front of democracies towards China, the final sessions will also bring in development chiefs and address global challenges including the Covid-19 pandemic and climate change. — AFP
President Joe Biden pledges that the United States would be ready to launch a vaccination campaign for 12 to 15-year-olds as soon as Pfizer's Covid shot is approved for the age group.
Biden says in an address from the White House that "if that announcement comes, we are ready to move immediately." — AFP
French Health Minister Olivier Veran on Tuesday defended the country's staggered rollout of Covid-19 vaccines over the coming weeks, despite calls for making more adults eligible immediately as some doses go unused.
Currently people aged 55 and older can get the jab, but several vaccination centres have reported a shortage of candidates and surplus vials in recent weeks.
That has prompted some local officials to quietly offer vaccines to all adults in a bid to speed up inoculations.
Since last weekend authorities are letting people aged 18 to 50 with chronic conditions such as high blood pressure or obesity to sign up as well, without having to present a medical certificate or doctor's prescription.
That raised the prospect that healthy adults would claim fake ailments in order to get the vaccine quicker, but Veran said the policy was simply "common sense."
"I trust the French," Veran told Europe 1 radio. "There will maybe be some attempts to circumvent the rules but it will be at the margins." — AFP
The United States is expected to authorize the Pfizer-BioNTech Covid-19 vaccine for children age 12 and up by early next week, US media reported Monday.
Pfizer has applied for emergency use authorization for its Covid vaccine for children and teenagers between ages 12 and 15, according to CNN, citing a government official.
"The FDA will have to amend the emergency use authorization for the vaccine, but the process should be straightforward," CNN reported. — AFP
Vaccine maker the Serum Institute of India is set to invest in facilities in Britain and could even manufacture inoculations in the UK in future, Prime Minister Boris Johnson said Monday.
Johnson's Downing Street office said the £240 million ($334 million) project would include a sales office, "clinical trials, research and development and possibly manufacturing of vaccines".
The Serum Institute of India (SII) is the world's largest vaccine maker by volume, and has been at the forefront of producing the lower-cost AstraZeneca coronavirus shot. — AFP
Hong Kong migrant worker groups criticize plans to make coronavirus vaccines compulsory for all foreign domestic helpers, labelling the move "discriminatory and unjust".
Health officials said they were planning to roll out mandatory inoculations for the 370,000 domestic helpers in the city, mostly poorly-paid women from the Philippines and Indonesia.
Those wanting to apply for work visas -- or renew their current ones -- would need to show they had been vaccinated, officials said Friday. — AFP
The World Health Organization says it had listed the anti-COVID-19 Moderna vaccine for emergency use.
The listing procedure helps countries unable to assess a vaccine's effectiveness themselves have access as quickly as possible and allow the Covax vaccine sharing scheme and other partners to distribute it to poorer countries.
The US vaccine is the fifth jab to earn WHO's emergency listing. — AFP
Brazil's national health regulator says Thursday its decision to reject the Russian-made Sputnik V vaccine was based on the developer's own data, after the latter threatened to sue for defamation.
The Brazilian agency, Anvisa, based the decision on evidence the vaccine carried a live version of a common cold-causing virus — information it says "was submitted by the Sputnik V vaccine developer itself."
The decision to deny emergency use authorization for the vaccine, announced Monday, has blown up into an all-out international row. — AFP
BioNTech says that it expected its COVID-19 vaccine, jointly developed with Pfizer, to be available to 12 to 15-year-olds in Europe from June.
The German firm's CEO Ugur Sahin tells Der Spiegel weekly that it was "in the final stretches" of preparing its submission for European regulatory approval.
The evaluation of the trial data "takes four to six weeks on average", he adds. — AFP
One dose of the Pfizer or AstraZeneca vaccines reduces the chances of someone infected with coronavirus from spreading it to other household members by up to 50 percent, according to an English study published Wednesday.
The Public Health England (PHE) research found that those who became infected three weeks after receiving their first jab were between 38 and 49 percent less likely to pass the virus on to their household contacts than those who were unvaccinated.
"This is terrific news — we already know vaccines save lives and this study is the most comprehensive real-world data showing they also cut transmission of this deadly virus," said British Health Secretary Matt Hancock. — AFP
The developers of Russia's Sputnik V coronavirus vaccine criticize Brazil's refusal to import the jab as politically motivated.
Brazilian health regulator Anvisa on Monday denied requests from several states to receive batches of Sputnik V, saying it did not have the data needed to verify the jab's safety and efficacy.
"Anvisa's delays in approving Sputnik V are unfortunately of a political nature and have nothing to do with access to information or science," the official Sputnik V Twitter account says. — AFP
The number of COVID-19 jabs administered globally surpassed the one billion mark on Saturday, offering hope even as the number of virus cases worldwide hit a new daily record mainly due to an explosion of infections in India.
At least 1,002,938,540 vaccine doses have been administered in 207 countries and territories, according to an AFP tally.
Nevertheless, the number of new infections topped 893,000 worldwide on Friday, a new daily record. — AFP
Johnson & Johnson Covid vaccinations can restart, US health regulators say, after the shots' rollout was paused due to worries over blood clotting.
Health authorities in the United States on April 14 proposed a halt on the vaccine following instances of severe blood clots among a handful of the millions of Americans who received the vaccine.
The news came shortly after an expert panel recommended lifting the pause because the shots benefits exceeded possible dangers. — AFP
Fake doses of the Pfizer coronavirus vaccine are being sold in Mexico and Poland for as much as $2,500 a shot, the US drugmaker and an official confirm.
At a clinic in Mexico some 80 people received bogus vaccines, which appeared to have been physically harmless though offered no protection against the potentially deadly disease ravaging the country, a report in the Wall Street Journal says.
Mexico's government spokesman on COVID-19 Hugo Lopez-Gatell said "no product was found that could affect" the health of those scammed, adding several people had been arrested. — AFP
French-Austrian vaccine developer Valneva announces that it had launched a Phase 3 trial of its candidate vaccine against COVID-19 -- the last testing stage before seeking regulatory approval.
The study, which has been dubbed "Cov-Compare", will compare how participants' immune systems respond to Valneva's VLA2001 vaccine with how they respond to AstraZeneca's coronavirus shot.
"Approximately 4,000 participants will receive two doses of either vaccine," says Valneva, adding that the study would be carried out at around 25 sites in the United Kingdom. — AFP
The EU will have enough COVID-19 vaccine doses to cover 70% of its adult population by mid-July due to higher production within the bloc, a senior official said Tuesday.
"Fifty-three factories are producing vaccines in the EU. Our continent is now the largest producer in the world after the United States," internal markets commissioner Thierry Breton told French daily Le Figaro in an interview.
"I am now certain of how many doses are currently in production and I know how many millions will be delivered each week," he said.
"This allows me to assure you that we well have by mid-July the number of doses necessary for vaccinating 70% of the European Union's adult population," he said, citing the threshold many health experts say is necessary to achieve "herd immunity". — AFP
The US Food and Drug Administration requests that production of the Johnson & Johnson coronavirus vaccine be halted at a factory that previously reportedly ruined about 15 million doses of the shot.
The pharmaceutical giant told AFP at the end of March it had identified a batch of doses at a plant in Baltimore run by Emergent BioSolutions "that did not meet quality standards," but did not confirm the specific number affected.
The New York Times later reported the batch consisted of about 15 million doses. — AFP
The European Union is very unlikely to renew its COVID-19 vaccine contracts with pharmaceutical company AstraZeneca, a French minister says.
Denmark this week banned the use of AstraZeneca jabs over blood clot concerns, just as the EU said it was expecting 50 million Pfizer vaccine doses earlier than expected.
No final EU decision had been taken, French Industry Minister Agnes Pannier-Runacher tells RMC radio, but "it is highly probable" that no further AstraZeneca doses would be ordered. — AFP
The head of Pfizer says in an interview that people will "likely" need a third dose of his company's COVID-19 shot within six to 12 months of vaccination, while elsewhere defending the relatively higher cost of the jab.
CEO Albert Bourla also says annual vaccinations against the coronavirus may well be required.
"We need to see what would be the sequence, and for how often we need to do that, that remains to be seen," Bourla tells CNBC in an interview. — AFP
A pause on all US vaccinations with the Johnson & Johnson Covid shot will continue for at least another week after members of a government-convened expert panel said Wednesday they needed more time to assess its possible links to a clotting disorder.
The Centers for Disease Control and Prevention (CDC) convened a meeting a day after authorities reported six cases of women developing brain clots along with low blood platelet counts, including one death, within two weeks of people getting the one-dose Covid-19 vaccine.
The shot has been given to some 7.2 million Americans, and participants at the meeting were told on Wednesday a seventh case involving a 28-year-old woman has been identified. — AFP
Russian President Vladimir Putin said Wednesday he has received the second dose of a vaccine against the coronavirus and said he hopes Russians follow his example.
"I want to inform you that right now, before entering this room, I also received the second vaccination," he said at a televised meeting. "I assume that you, taking care of yourself and your loved ones, will do the same and follow my example." — AFP
A leading local drugmaker says India has authorized the Sputnik V COVID-19 vaccine in a boost for the nation's inoculation drive as infection rates soar to record highs.
Russia's Sputnik V is the third vaccine to be approved by India after the Oxford-AstraZeneca vaccine and Covaxin, which was developed by Indian firm Bharat Biotech.
"We are very pleased to obtain the emergency use authorisation for Sputnik V in India," says G.V. Prasad, co-chair of pharmaceutical company Dr Reddy's Laboratories, in a statement. — AFP
Britain says it had met its target to offer by April 15 a coronavirus vaccine first dose to all over-50s, the clinically vulnerable and health and social care workers.
"We have now passed another hugely significant milestone in our vaccine programme by offering jabs to everyone in the nine highest risk groups," Prime Minister Boris Johnson says in a statement. — AFP
Britain says late Monday it had met its target to offer by April 15 a coronavirus vaccine first dose to all over-50s, the clinically vulnerable and health and social care workers.
"We have now passed another hugely significant milestone in our vaccine programme by offering jabs to everyone in the nine highest risk groups," Prime Minister Boris Johnson says in a statement. — AFP
Venezuela hopes to produce two million doses per month of a Cuban coronavirus vaccine, President Nicolas Maduro said Sunday.
The South American country will also take part in Phase 3 trials for the Abdala vaccine produced by its socialist ally.
"We've signed an agreement to produce in our laboratories... two million vaccines a month of the Abdala vaccine... for August, September, approximately," said Maduro in a television address. — AFP
Cameroon received a gift of 200,000 doses of China's Sinopharm vaccine against Covid-19 on Sunday, state television reports.
The vaccine will be used in the first phase of the central African country's inoculation campaign.
Health Minister Malachie Manaouda urged Cameroonians to get the jab, "especially priority targets", in a statement dated Friday. — AFP
Europe's stuttering vaccine rollout faces multiple hurdles as EU regulators say they were reviewing side effects of the Johnson & Johnson shot and France further limits its use of the AstraZeneca jab.
The US drugs regulator says it had not found a "causal" link between the J&J vaccine and blood clots, but that its probe was continuing after "a few individuals" suffered complications.
Much of the world is still in the clutches of the pandemic that has killed 2.9 million people, from Brazil, where the virus is killing more than 4,000 people a day, to Japan where the government has tightened restrictions once again. — AFP
Hong Kong confirms it has requested AstraZeneca suspend delivery of its Covid-19 vaccine amid fears of severe side effects and concerns over its efficacy against new variants of the coronavirus.
Hong Kong's health chief Sophia Chan said the city has asked AstraZeneca not to deliver as planned later this year.
"We think it is not necessary for AstraZeneca to deliver the vaccines to the city within this year," she says, adding Hong Kong wanted "to avoid any waste as vaccines are in short supply globally". — AFP
Australia on Thursday joined a growing number of countries halting the use of the AstraZeneca coronavirus vaccine for younger people over fears it can cause serious blood clots.
In a further setback for Australia's already halting coronavirus vaccine rollout, officials said the AstraZeneca shot should no longer be given to people under the age of 50, unless they had already received a first dose without any ill effects.
Prime Minister Scott Morrison held an evening press conference to announce the decision shortly after the government's medical advisory board decided to follow European and other countries in limiting the use of AstraZeneca.
"It has not been our practice to jump at shadows, it has not been our practice to take unnecessary precautions," he said in explaining the step.
"We've been taking the necessary precautions based on the best possible medical advice." -- AFP
The EU's medicines regulator says that blood clots should be listed as a rare side effect of the AstraZeneca jab but the benefits continue to outweigh risks, as several countries battle fresh virus surges amid vaccine shortfalls.
A number of nations have suspended the use of AstraZeneca's vaccine for younger populations after it was earlier banned outright in several places over blood clot scares.
The United Kingdom says it will adopt new medical advice to offer most people under 30 an alternative to AstraZeneca if possible, after reporting 19 deaths from clots among people who received the shot. — AFP
Britain begins rolling out its third coronavirus vaccine, from US company Moderna, as questions mount over jabs from the country's main supplier, AstraZeneca.
The Moderna vaccine, which is already being delivered in Europe and the United States, joined ones from AstraZeneca-Oxford University and Pfizer-BioNTech in Britain's armoury against COVID-19.
The first jabs of the two-stage Moderna inoculation were injected at a hospital in Wales, in a timely diversification of Britain's rollout that was hailed by Prime Minister Boris Johnson. — AFP
President Joe Biden announces that all adults across America will be eligible for COVID-19 vaccines within two weeks, as economic powerhouse California set a June 15 target to fully reopen businesses.
The positive news from the United States -- which has reported the most coronavirus deaths of any country but is now a leader in vaccine distribution -- contrasted with a record daily toll in Brazil and Europe's troubled rollout of the AstraZeneca shot.
Biden announces in a White House speech that he is moving up the deadline for all over 18s to be eligible for vaccines to April 19. The previous target had been May 1. — AFP
The United States will soon be able to ramp up vaccine assistance abroad and will not seek "favors in return," Secretary of State Antony Blinken says, putting a veteran aid administrator in charge.
Blinken says the top US priority was to fight COVID-19 at home but that the goal would be achieved soon, pointing to President Joe Biden's promise that 90 percent of Americans will be near a vaccination site by April 19.
"We are exploring options to share more with other countries going forward. We believe that we'll be in a position to do much more on this front," Blinken says. — AFP
The Centers for Disease Control and Prevention says the United States has administered at least one dose of coronavirus vaccine to more than 100 million people.
The 101,804,762 people who have received at least one dose represents more than 30% of the US population, according to CDC data. — AFP
Chinese biopharmaceutical firm Sinovac says a third production line for its COVID-19 vaccine has been put into use, doubling its annual capacity of the jabs to two billion doses.
Its CoronaVac is one of four domestic vaccines given conditional approval by Chinese authorities, which helps rush emergency drugs to market.
On Wednesday, experts from the World Health Organization said an interim analysis of clinical trial data from two Chinese vaccines, including Sinovac's product, showed they demonstrated "safety and good efficacy", although more data is still needed. — AFP
Hong Kong will resume administering the Pfizer/BioNTech coronavirus jab on Monday after the pharma giant said a packaging flaw that temporarily halted its use did not affect its safety, officials say.
The financial hub suspended use of the German-made vaccine last month when Fosun, its China distributor, informed authorities that some vial caps were defective.
It was a blow to the roll-out of mass vaccination programmes against a deadly virus that has killed more than 2.7 million people around the world and hammered the global economy. — AFP
Germany expects to still offer every adult a coronavirus jab by the end of the summer, Health Minister Jens Spahn says Tuesday, despite new guidelines against the use of AstraZeneca for under-60s in the country.
"If delivery pledges are kept to and all vaccines receive their approvals as planned," then Europe's biggest economy would be able to vaccinate its population "by the end of summer", says Spahn. — AFP
Cuba begins vaccinating tens of thousands of health care workers with a second COVID-19 vaccine, even though it has yet to complete clinical trials.
Last week, Cuba started vaccinating 150,000 health care workers with its Soberana 2 vaccine that is still in the third phase of clinical trials.
And on Monday, the island nation started giving its Abdala vaccine to 124,000 health care workers -- Abdala is likewise still in phase 3 of COVID vaccine trials. — AFP
Venezuelan President Nicolas Maduro on Sunday offered "oil for vaccines" as his country, which is under economic sanctions affecting the oil sector in particular, faces a second wave of coronavirus.
"Venezuela has the oil tankers, it has customers ready to buy oil from us. It would devote part of its production to obtain the vaccines it needs. Oil for vaccines!" said Maduro during an appearance on public television.
Venezuela has so far only authorized the use of the Russian Sputnik V vaccine and the one produced by Chinese company Sinopharm. — AFP
Chile is a world leader in its coronavirus vaccination program and has already given at least one dose to almost a third of its population.
By Thursday the narrow South American nation, hemmed in by the Andes mountains and the Pacific ocean, had given more than six million people a single dose and 3.1 million both doses, including most over-70s.
And yet that same day, the government put more than 80% of the country's 19 million people in lockdown. — AFP
The European Union will look for ways to end its vaccine struggles at a summit on Thursday.
AstraZeneca's jab is also at the centre of the EU's vaccine woes, with an infuriated Brussels tightening export controls after the firm failed to deliver the doses it had promised to the bloc.
EU leaders will meet via videoconference on Thursday to discuss AstraZeneca supplies, as well as new vaccine export rules that will weigh how needy countries are in terms of infection rates, how many jabs they have, and how readily they export doses to the bloc. — AFP
British-Swedish drugmaker AstraZeneca revises down by three percentage points the effectiveness of its COVID-19 vaccine after American authorities raised concerns that results reported from its US trial were outdated.
The company now says its vaccine is 76 rather than 79% effective at preventing any kind of symptomatic COVID-19.
It remains 100% effective against severe COVID-19, it adds. — AFP
Hong Kong health authorities have ejected a private clinic from the city's coronavirus vaccination program after it reportedly recommended the German-made Pfizer/BioNTech shot to patients over the one from China's Sinovac.
The move illustrates the Hong Kong government's sensitivity to any criticism of the Sinovac vaccine, which has a comparatively lower efficacy rate and was fast-tracked by regulators despite a lack of published data.
The city's health department said Tuesday that the clinic would no longer administer COVID-19 jabs because a doctor violated an agreement with the inoculation program. — AFP
Hong Kong and Macau suspend the use of Pfizer/BioNTech's coronavirus vaccine on Wednesday after being informed of a packaging problem affecting one batch of vials, while stressing they did not believe there was a safety risk.
The stoppage is the latest blow in efforts to role out mass vaccination programmes against a deadly virus that has killed more than 2.7 million people around the world and hammered the global economy.
"For the sake of precaution, the current vaccination must be suspended during the period of investigation," Hong Kong's government says in a statement. — AFP
Pharmaceutical giant AstraZeneca said Monday that trials showed its Covid-19 vaccine is 100 percent effective in preventing severe disease, as a row simmered between Britain and the EU over much-needed supplies of the jab.
The AstraZeneca shot is cheaper and easier to store than many of its rivals, but several countries in Europe and other parts of the world last week suspended its use because of isolated cases of blood clots.
It is also at the centre of a row between Britain and the EU, after Brussels threatened to ban exports to the UK unless the company delivers more of the 90 million doses it agreed to supply in the first quarter of 2021. — AFP
German Chancellor Angela Merkel voices support for EU chief Ursula von der Leyen's threat to block AstraZeneca vaccines produced in the bloc from being exported, ahead of a crunch EU summit on the escalating row.
"I support Commission President Ursula von der Leyen," says Merkel.
"We have a problem with AstraZeneca," she adds. — AFP
Pharmaceutical giant AstraZeneca say that US trials showed its Covid-19 vaccine was 100 percent effective in preventing severe disease, even as a poll showed trust in the jab had plunged in many European countries. — AFP
EU chief Ursula von der Leyen threatens to halt exports of AstraZeneca's Covid-19 vaccines if the bloc does not receive its promised deliveries first.
The warning comes as the European Union struggles to speed up its inoculation campaign, with many member states facing a third coronavirus wave and renewed curbs on public life. — AFP
More than 425 million doses of Covid-19 vaccines have been administered worldwide, according to an AFP tally based on official sources at 1800 GMT on Saturday.
At least 162 countries or territories, accounting for 93 percent of the global population, have begun rolling out their vaccination programmes. But so far, 58 percent all doses administered have been in "high-income" countries, where just 16 percent of the world's inhabitants live. — AFP
The Russian Direct Investment Fund (RDIF), Russia’s sovereign wealth fund thanks the government of Mexico, its customs and armed forces for confiscating a batch of fake Russian Sputnik V COVID-19 vaccines.
Earlier Friday, Mexican authorities seized a batch of vaccines designed and packaged as Sputnik V.
RDIF says that every manufactured batch of Sputnik V COVID-19 vaccine is undergoing strict controls. Each vial has a unique QR-code, allowing RDIF and partners to trace it to the place of origin.
Anglo-Swedish pharmaceutical giant AstraZeneca on Thursday welcomes verdicts given by the health regulators of the European Union and Britain finding its Covid-19 vaccine is safe and effective.
"Vaccine safety is paramount and we welcome the regulators' decisions which affirm the overwhelming benefit of our vaccine in stopping the pandemic," AstraZeneca's chief medical officer says in a statement. — AFP
Britain insisted Thursday that its plan to ease coronavirus lockdowns in the coming months remained on track, despite a vaccine supply shortfall from India which will hit the inoculation drive in April.
The state-run National Health Service in England has warned in a letter to local vaccination centers that doses will be "significantly constrained" from March 29 for four weeks.
The next phase of the inoculation campaign, covering people in their 40s, will have to be suspended until May, the letter said.
The problem is linked to a delay in getting new jabs from India by UK-based drugs giant AstraZeneca — the same company whose supply issues have caused anger in the European Union.
"It's a very complex international supply chain and that does mean occasionally we will experience issues and that's what we've experienced right now," cabinet minister Robert Jenrick told Sky News.
But the government was sticking to its target of offering a first dose to every adult by the end of July, ministers said, after Britain on Wednesday said it had surpassed 25 million jabs delivered. — AFP
The World Health Organization says Wednesday its experts are still reviewing safety data on the AstraZeneca COVID-19 vaccine following concerns around blood clots but recommended that vaccination programs continue.
"The WHO Global Advisory Committee on Vaccine Safety is carefully assessing the latest available safety data," the UN health agency said in a statement.
"At this time, WHO considers that the benefits of the AstraZeneca vaccine outweigh its risks and recommends that vaccinations continue." — AFP
Former president Donald Trump encourages his Republican supporters -- one of the main groups resistant to COVID-19 vaccines -- to get their shots.
"I would recommend it," Trump says during an interview on Fox News.
"I would recommend it to a lot of people that don't want to get it and a lot of those people voted for me, frankly," he says. — AFP
US manufacturer Moderna on Tuesday said it has started COVID-19 vaccine trials for children aged from 6 months to under 12 years old, with plans to enroll about 6,750 participants.
"We are pleased to begin this Phase 2/3 study of mRNA-1273 in healthy children in the US and Canada," said CEO Stephane Bancel in a statement.
"This pediatric study will help us assess the potential safety and immunogenicity of our COVID-19 vaccine candidate in this important younger age population."
US health authorities say that fewer children have been sick with COVID-19 compared to adults, but they can be infected and can spread the virus.
Most infected children have mild symptoms or no symptoms at all. — AFP
Venezuela announces that it would not authorize AstraZeneca's COVID-19 vaccine, as the three largest European countries suspended their rollouts of the jab.
"Venezuela will not authorize the use of the AstraZeneca vaccine in the process of immunizing our population due to complications" in vaccinated patients, vice president Delcy Rodriguez says on public television. — AFP
Latvian health authorities said Monday they were temporarily suspending the use of AstraZeneca's Covid-19 vaccine, following the lead of other countries to have paused rollouts over blood clot fears.
Latvia's "health authorities are asking doctors not to use the opened vials of the AstraZeneca vaccine and not to open new ones," the Baltic state's health agencies said in a joint statement.
They said they were halting the use of the vaccine "as a precautionary measure" based on reports of side effects from other EU countries, while adding that no such cases have been confirmed in Latvia. — AFP
Indonesia will delay the rollout of AstraZeneca's COVID-19 jab pending a review by the World Health Organization (WHO) into blood clot fears, the Southeast Asian nation's health minister said Monday.
"To be conservative, (Indonesian health regulators) are delaying the implementation of Astrazeneca while waiting for confirmation from WHO," Budi Gunadi Sadikin said. —AFP
Dutch health officials say they had suspended the use of AstraZeneca's coronavirus vaccine on Sunday for two weeks after "possible side effects" were reported in Denmark and Norway.
"Based on new information, the Dutch Medicines Authority has advised, as a precautionary measure and pending further investigation, to suspend the administration of the AstraZeneca vaccine," the Health Ministry says in a statement.
"The crucial question is whether these are complaints after or because of the vaccination. There should be no doubt about the vaccines," Health Minister Hugo de Jonge says in the statement. — AFP
Anglo/Swedish pharmaceutical giant AstraZeneca announces a fresh shortfall in planned vaccine shipments to the European Union, citing production problems and export restrictions.
"AstraZeneca is disappointed to announce a shortfall in planned COVID-19 vaccine shipments to the European Union (EU) despite working tirelessly to accelerate supply," it says in a statement.
The company had previously warned it was facing shortfalls from its European supply chain due to "lower-than-expected output from the production process." — AFP
Brazil grants full regulatory approval to the COVID-19 vaccine developed by Oxford University and pharmaceutical firm AstraZeneca, voicing confidence in it even as a raft of countries suspended its use.
Federal health regulator Anvisa says it saw "no health risk for the population associated with the use of this vaccine," and upgraded it from emergency to full regulatory approval.
"The benefits outweigh the risks," it says. — AFP
Health Minister Jens Spahn says that Germany would have to wait until "mid-to-late April" for the newly approved Johnson & Johnson coronavirus vaccine, adding that the EU is querying the company over the delays.
Johnson & Johnson's single-dose COVID-19 vaccine on Thursday became the fourth jab to be authorized for use in the European Union. — AFP
Thailand abruptly delays its roll-out of the AstraZeneca Covid-19 vaccine on Friday, stopping Premier Prayut Chan-O-Cha from getting the first jab as several European nations suspended their programmes over blood clot fears.
The kingdom was scheduled to start administering the Oxford/AstraZeneca vaccine on Friday, with Prayut expected to be filmed receiving the first injection.
"Vaccine injection for Thais must be safe, we do not have to be in a hurry," Piyasakol Sakolsatayadorn, an adviser for the country's Covid-19 vaccine committee, tells a press conference. — AFP
A health official says Thailand has delayed its roll-out of the AstraZeneca COVID-19 vaccine, after several European nations suspended their programmes over blood clot fears.
"Though the quality of AstraZeneca is good, some countries have asked for a delay," says Piyasakol Sakolsatayadorn, an advisor for the country's Covid-19 vaccine committee, in a press conference.
"We will delay (as well)". — AFP
Denmark, Norway and Iceland on Thursday temporarily suspended the use of AstraZeneca's Covid-19 vaccine over concerns about patients developing post-jab blood clots, as the manufacturer and Europe's medicines watchdog insisted the vaccine was safe.
The European Medicines Agency (EMA) told AFP that information available so far indicated the risk of blood clots in those vaccinated against Covid-19 was "no higher than that seen in the general population."
It also said that European countries could keep using the AstraZeneca vaccine while the issue was investigated, concluding that "the vaccine's benefits continue to outweigh its risks". — AFP
The European Union will receive an extra four million BioNTech/Pfizer vaccine doses over the next two weeks to be deployed to Covid-19 "hotspots", European Commission chief Ursula von der Leyen said Wednesday.
The delivery — over and above already agreed supplies from the vaccine-maker — will go to affected border regions within the bloc to "help ensure or restore free movement of goods and people", she said in a statement.
The announcement came as the commission attempted to persuade at least six member states — including her home country Germany — to lift virus-related border restrictions deemed by Brussels to be excessive.
It also follows a trip by the leaders of Austria and Denmark to Israel to form a vaccine-producing alliance that exemplified broad criticism of the lack of deliveries so far under the commission's pre-purchasing scheme. — AFP
The Red Cross warns Wednesday of a glaring gap in the plans to roll out Covid-19 vaccines around the world, saying remote communities risked missing out.
The International Federation of Red Cross and Red Crescent Societies is aiming to help vaccinate 500 million people against the disease.
The IFRC, which calls itself the world's largest humanitarian network, is planning to throw its expertise into the distribution and acceptance of vaccines among some of the hardest-to-reach communities. — AFP
There was no special treatment for Italian President Sergio Mattarella when he received his coronavirus vaccine in a Rome hospital on Tuesday.
In a photo issued by his office, the 79-year-old head of state was pictured sitting in a chair alongside numerous others — all socially distanced — in a large room in which several medics were working.
Italy began its vaccination campaign in late December, but like many other countries, the program has stalled due to supply hiccups.
Mattarella waited until his age group was called up before getting the Moderna vaccine at Rome's Spallanzani hospital.
He was vaccinated as part of "the campaign for those born in 1941", his office said in a statement. — AFP
The United States denounces what it called a Russian disinformation campaign against US-made COVID-19 vaccines, saying Moscow was putting lives at risk.
The Global Engagement Center, an arm of the State Department whose activities include monitoring foreign propaganda, says that Russian intelligence was behind four online platforms involved in a campaign. — AFP
The EU's drug watchdog says on Thursday it had started a "rolling review" of Russia's Sputnik V coronavirus vaccine, a key step towards approval for use across the 27-nation bloc.
"EMA has started a rolling review of Sputnik V, a Covid-19 vaccine developed by Russia's Gamaleya National Centre of Epidemiology and Microbiology," the Amsterdam-based European Medicines Agency (EMA) says in a statement. — AFP
A shipment of 3.94 million coronavirus jabs arrived in Nigeria on Tuesday, making Africa's most populous nation the world's third country to receive vaccines under Covax, a global scheme to provide free inoculations, an AFP journalist saw.
The Oxford/AstraZeneca vaccines, manufactured by the Serum Institute of India, are the first of 16 million doses that Covax plans to deliver over the coming months to Nigeria, where they will be given first to healthworkers, the government said. — AFP
The health ministry announces that Iraq received 50,000 Sinopharm vaccines donated by China, launching a long-awaited vaccination campaign.
Health ministry spokesman Seif al-Badr told reporters that the first delivery in the early hours meant inoculations could begin.
"The doses will be delivered to Baghdad's three main hospitals, and maybe to some provinces," says Badr, who confirmed the jabs were donations. — AFP
Ghana's President Nana Akufo-Addo on Monday became the first recipient of a coronavirus vaccine under the global Covax scheme, as US health workers prepared to distribute nearly four million doses of the single-shot Johnson & Johnson jab.
Covax, a scheme designed to ensure that poorer countries do not miss out on Covid-19 vaccines that have so far been largely hoovered up by rich nations, is aiming to deliver at least two billion doses by the end of the year.
Akufo-Addo received his AstraZeneca shot live on television along with his wife, a day before the rest of the first batch of 600,000 Covax doses are deployed across Ghana.
"It is important that I set the example that this vaccine is safe by being the first to have it, so that everybody in Ghana can feel comfortable about taking this vaccine," the president said. — AFP
Four million doses of the latest Covid-19 vaccine to get US approval will be delivered across the country as early as Tuesday, a senior administration official said.
The United States on Saturday authorized Johnson & Johnson's Covid vaccine for emergency use, boosting President Joe Biden's plan to battle the outbreak that has killed more than 500,000 Americans.
The single-shot vaccine — the third type to be authorized — is highly effective in preventing severe Covid-19, including against newer variants, the Food and Drug Administration (FDA) said before giving it a green light. — AFP
Willingness to get a COVID-19 vaccine is on the rise compared to last year, a survey of six industrialised countries published on Monday shows.
More people in the United Kingdom, the United States and even vaccine-sceptical France now accept the idea of getting a coronavirus jab, KekstCNC, an international consultancy, says in the survey conducted in February.
The survey also covers Germany, Japan and Sweden where a similar trend was clear, it says.
"As vaccine rollouts commence, higher numbers of people in all countries say they would take the vaccine," the study says. — AFP
Thousands of people demonstrated in cities across Argentina on Saturday to protest the "VIP vaccinations" scandal that forced the health minister to resign.
Gines Gonzalez Garcia quit a week ago at the president's request after it emerged that his friends had been able to skip the line for coronavirus inoculation.
Protesters carrying signs reading "Give me my vaccine" and "Stop wasting our money" gathered outside the government headquarters in Plaza de Mayo in Buenos Aires.
"They started by vaccinating friends of the government. It is not appropriate. They are stealing someone else's life," protester Irene Marcet told AFP.
Since Argentina began vaccinating its people, only healthcare workers had received the jab until Wednesday, when over-70s in Buenos Aires province were also invited to be immunized.
On Monday, the government released a list of 70 people who received the vaccine outside of the official campaign, which included the 38-year-old economy minister and former president Eduardo Duhalde, his wife and their children. — AFP
The United States on Saturday authorized Johnson & Johnson's Covid vaccine for emergency use, giving the nation a third shot to battle the outbreak that has killed more than 500,000 Americans.
The single-shot vaccine is highly effective in preventing severe Covid-19, including against newer variants, the Food and Drug Administration (FDA) said before giving it a green light.
"This is exciting news for all Americans, and an encouraging development in our efforts to bring an end to the crisis," US President Joe Biden said in a statement. — AFP
A US panel of independent experts vote unanimously in favor of recommending Johnson & Johnson's one-dose COVID-19 shot for emergency approval, clearing the way for a third vaccine to soon begin shipping in the world's hardest hit country.
The committee's 22 members were convened by the Food and Drug Administration and included leading scientists as well as consumer and industry representatives.
Although their recommendations aren't binding, they are usually followed. — AFP
The United States hails progress in turning around its troubled COVID-19 vaccine rollout, as the European Union says it was on track to meet jab targets and Asia's inoculation drive gained pace on Friday.
Brazil hit 250,000 fatalities -- the second-highest national death toll after the United States -- while the worldwide vaccine campaign received the endorsement of Queen Elizabeth II, 94, who urged people not to be wary of the shot.
President Joe Biden declared the US rollout is now "weeks ahead of schedule" as he celebrated 50 million doses administered since he took office on January 20, but he warned Americans to keep masking up. — AFP
A US panel of independent experts is set to vote Friday on whether to recommend emergency approval of Johnson & Johnson's single-shot Covid-19 vaccine, potentially paving the way for at least three million doses to ship next week.
The committee's 22 members, who were convened by the Food and Drug Administration and include leading scientists as well consumer and industry representatives, will hold a daylong virtual meeting to decide if the known benefits of the drug outweigh its risks.
It is an exercise in transparency without parallel among other advanced countries, giving the public access to the nitty gritty details of the scientific debate. — AFP
Ghana receives the first shipment of COVID-19 vaccines from Covax, a global scheme to procure and distribute inoculations for free, as the world races to contain the pandemic.
Covax, launched last April to help ensure a fairer distribution of coronavirus vaccines between rich and poor nations, said it would deliver two billion doses to its members by the end of the year. — AFP
The world's biggest vaccine maker, India's Serum Institute, has urged other countries to be "patient" about it supplying anti-coronavirus shots, saying it has been instructed to prioritize its home market.
"Dear countries & governments, as you await #COVISHIELD supplies, I humbly request you to please be patient," Serum chief Adar Poonawalla tweeted on Sunday.
"@SerumInstIndia has been directed to prioritise the huge needs of India and along with that balance the needs of the rest of the world. We are trying our best."
Serum, from its sprawling facility in Pune in western India, is producing hundreds of millions of doses of the AstraZeneca vaccine.
Many countries around the world, particularly poorer nations, are relying heavily on the company for supplies of the vaccine, and it has already shipped millions of doses abroad. — AFP
Australia launches its public rollout of the COVID-19 vaccine Monday amid protests over the campaign, including a vocal show of opposition by crowds at the final of the Australian Open.
Some 60,000 doses are set to be given this week, starting with frontline workers — from healthcare staff to hotel quarantine employees and police — and residents of aged care homes.
Morning television news programmes show the first shots being administered to medical and quarantine staff in Melbourne and Sydney, a day after Prime Minister Scott Morrison received his jab in a "curtain-raiser" event aimed at convincing Australians the vaccine was safe. — AFP
The number of coronavirus vaccine doses administered worldwide passed 200 million Saturday, an AFP count shows, as wealthy G7 countries pledged to more than double aid to support access for the less well-off.
With 45% of injections so far among the rich club -- which accounts for just 10 percent of the global population -- the G7 on Friday said its aid to projects like the World Health Organization's Covax now amount to $7.5 billion.
Moscow announces it had registered its third vaccine against the coronavirus and promised to introduce the jab to the Russian population by March.
Russia was the first country to register a vaccine against COVID-19 in August ahead of clinical trials, and the Sputnik V jab has been authorised in more than two dozen countries around the world.
"Today we note that a third vaccine, CoviVac, has been registered," Prime Minister Mikhail Mishustin says at a government meeting broadcast on state television. — AFP
Britain has circulated a draft resolution to members of the UN Security Council calling on rich countries to donate doses of the COVID-19 vaccine to poorer and war-torn states, according to a text of the draft seen by AFP Friday.
The resolution, submitted Thursday by Britain to the other 14 members of the Security Council, "emphasizes the need for solidarity, equity, and efficacy and invites donation of vaccine doses from developed economies to low- and middle-income countries and other countries in need."
US President Joe Biden uses a visit to a Pfizer factory manufacturing coronavirus vaccines to reassure Americans that the shots are safe and hold the key to beating the pandemic.
Biden, who has made getting COVID-19 under control his top task since taking office a month ago, addresses Americans skeptical about the medicines, which were produced at record speed in response to the global health crisis.
"The vaccines are safe. Please, for yourself, your family, your community, this country, take the vaccine when it's your turn and it's available. That's how we beat this pandemic," he says against a backdrop of Pfizer's gleaming manufacturing equipment at the Michigan facility. — AFP
Germany's BioNTech says it still intends to provide Taiwan with coronavirus vaccine doses after the island's health chief warned "political pressure" had scuppered a deal with the company.
Taiwanese health minister Chen Shih-chung said Wednesday that negotiations with the German firm to acquire five million Pfizer/BioNTech shots fell through in December "because someone doesn't want Taiwan to be too happy".
His comments raised concerns China might be trying to hinder Taiwan's inoculation drive. — AFP
Britain will on Wednesday call on the UN Security Council to push for temporary ceasefires in conflict zones to enable the "moral duty" of rolling out vaccines against the coronavirus.
Britain holds the council's chair this month and Foreign Secretary Dominic Raab said his resolution would also demand "equitable access" around the world to vaccines against the pandemic.
"Global vaccination coverage is essential to beating coronavirus," he said in a statement, stressing the need for temporary ceasefires to help inoculate more than 160 million people at risk in conflict zones such as Yemen, South Sudan, Somalia and Ethiopia. — AFP
The head of the EU's disease control agency warns that the novel coronavirus could last indefinitely even as global infections slowed by nearly half in the last month and vaccine rollouts gathered pace in parts of the world.
In an interview with AFP, ECDC chief Andrea Ammon urges European countries in particular not to drop their guard against a virus that "seems very well adapted to humans" and may require experts to tweak vaccines over time, as is the case with the seasonal flu.
"So we should be prepared that it will remain with us," according to Ammon, head of the Stockholm-based European Centre for Disease Prevention and Control. — AFP
US biotech firm Moderna says it was seeking clearance with regulators around the world to put 50% more coronavirus vaccine into each of its vials as a way to quickly boost current supply levels.
The company issued a statement after The New York Times first reported the US Food and Drug Administration had already cleared it to increase levels by 40 percent.
"In order to better maximize resources as well as maximize opportunities to deliver more doses into each market faster, Moderna has proposed filling vials with up to 15 doses of vaccine versus the previous 10 doses," a spokesperson says in a statement to AFP.
US President Joe Biden says Thursday the government had signed deals to acquire 200 million more COVID-19 vaccine doses.
"Just this afternoon, we signed ... final contracts for 100 million more Moderna and 100 million more Pfizer vaccines," he says after touring the National Institutes of Health near Washington.
"We're now on track to have enough supply for 300 million Americans by the end of July," he adds. — AFP
South Africa will begin its coronavirus inoculation campaign with Johnson & Johnson vaccines, the health minister says, after withholding the Oxford/AstraZeneca formula over doubts about effectiveness.
The country worst-hit by the pandemic in Africa has suspended its vaccine rollout -- meant to begin with Oxford/AstraZeneca this week -- after scientists found the shot failed to prevent mild and moderate illness caused by a local virus variant known as 501Y.V2.
"Given the outcomes of the efficacy studies..., (government) will continue with the planned phase one vaccination using the Johnson & Johnson vaccines instead of the AstraZeneca vaccine," Health Minister Zweli Mkhize tells a press briefing. — AFP
Peru begins its coronavirus immunization program just two days after receiving 300,000 vaccine doses from state-owned Chinese company Sinopharm.
The country has been hard hit by a second wave of the coronavirus pandemic sweeping Latin America.
It has recorded around 1.2 million cases and more than 42,000 deaths from COVID-19 among its population of 33 million. — AFP
The first 100,000 doses of Russia's Sputnik V vaccine will arrive in Venezuela next week, the South American nation's President Nicolas Maduro says.
"When the vaccination process begins, we are going to vaccinate all medical personnel, all health personnel in Venezuela," Maduro says in a televised address.
"The most vulnerable sectors, and then we will vaccinate the teachers." — AFP
South Africa says it would suspend the start of its COVID-19 vaccinations with the AstraZeneca jab after a study showed the drug failed to prevent mild and moderate cases of the virus variant that has appeared in the country.
Africa's hardest-hit nation was due to start its campaign in the coming days with a million doses of the vaccine developed by AstraZeneca and Oxford.
The suspension marks an important setback for the country, but officials said vaccine deliveries from other producers would soon be available and allow the campaign to move forward.
"It's a temporary issue that we have to hold on AstraZeneca until we figure out these issues," Health Minister Zweli Mkhize tells reporters during a virtual press conference. — AFP
China's drug authorities have given "conditional" market approval for a second COVID-19 vaccine, Sinovac's CoronaVac jab, the pharmaceutical company says.
The approval comes after multiple domestic and overseas trials of the vaccine in countries including Brazil and Turkey, although "efficacy and safety results need to be further confirmed", Sinovac says in a statement. — AFP
Europe and pharma groups must work together to speed up COVID-19 vaccinations, the head of the European branch of the World Health Organization says, expressing concern about the effectiveness of vaccines on virus variants.
"We need to join up to speed up vaccinations," WHO Europe director Hans Kluge tells AFP in an interview, as Europe bids to overcome a slow start to its vaccination campaign amid tensions between Brussels and vaccine manufacturers.
"Otherwise competing pharmaceutical companies (must) join efforts to drastically increase production capacity ... that's what we need," Kluge says.
Pharmaceuticals giant Johnson & Johnson says it submitted an application for emergency authorization of its COVID-19 vaccine with US health authorities.
The process could take several weeks, but if approved, the vaccine would be the third authorized in the United States, after those of Pfizer-BioNTech and Moderna.
J&J subsidiary Janssen Biotech "has submitted an application to the US Food and Drug Administration requesting Emergency Use Authorization for its investigational single-dose Janssen COVID-19 vaccine candidate," a company statement reads. — AFP
The Red Cross launches a plan Thursday to help vaccinate 500 million people against COVID-19 in over 100 countries, warning that leaving out the world's poorest could seriously backfire.
The International Federation of Red Cross and Red Crescent Societies said it would throw its weight into the distribution and acceptance of vaccines among some of the hardest-to-reach communities.
The Geneva-based IFRC said it would spend 100 million Swiss francs ($111 million, 92.5 million euros) on the push and was already working with governments in more than 60 countries to see where its help could be the most effective. — AFP
New York's Yankee Stadium will open as a coronavirus vaccination center later this week, but only for nearby residents to ensure the underprivileged receive doses, Mayor Bill de Blasio announced Wednesday.
The baseball ground — located in the Bronx — will only vaccinate people who live in that borough, one of poorest in the city and which has some of the highest rates of COVID-19 infections.
"It's a home run for justice and equity," de Blasio wrote on Twitter, using a baseball term.
The vaccinations will be by appointment, with 15,000 available in the first week. — AFP
Russia is working to increase production of its Sputnik V coronavirus vaccine in foreign countries, the Kremlin said Wednesday, as many European nations struggle to roll out their jabs.
"In the very near future there are plans to establish production in foreign countries, which will satisfy the demand from more and more countries," Kremlin spokesman Dmitry Peskov told reporters.
The comments come a day after The Lancet medical journal published results showing Sputnik V — named after the Soviet-era satellite — to be safe and 91.6 percent effective, allaying concerns over transparency. — AFP
A French lab will start producing Moderna's COVID-19 vaccine in March, while another will begin making the vaccine from Pfizer and BioNTech in April, Industry Minister Agnes Pannier-Runacher says.
President Emmanuel Macron pledged Tuesday that four sites on French soil would begin making coronavirus vaccines soon, as the government draws sharp criticism over an innoculation drive that has started off slowly.
French pride has also taken a hit after its pharma giant Sanofi said its Covid vaccine would not be ready until later this year. — AFP
Mexico approves Russia's Sputnik V coronavirus vaccine for emergency use in the country, one of the worst hit by the pandemic, following the release of positive trial results.
The move is a boost to the Latin American nation's efforts to keep its immunization program on track in the face of limited supplies from other manufacturers.
Regulatory agency Cofepris "has just granted authorization for the emergency use of the Sputnik V vaccine," deputy health minister Hugo Lopez-Gatell tells a news conference. — AFP
All elderly people living in English care homes who are eligible for the coronavirus vaccine have now been offered their first injection, the National Health Service (NHS) announces on Monday.
"The NHS vaccination programme, nurses, GPs and other NHS staff have offered the life-saving jab to people living at more than 10,000 care homes with older residents," says NHS England.
A small number of homes have had their visits deferred due to severe local outbreaks, and will be seen shortly, while some residents were unable to receive their first dose "for other clinical reasons", it adds. — AFP
The European Union backtracks on a threat to restrict exports of coronavirus shots to Northern Ireland in its growing row with Britain, as the WHO warned against "vaccine nationalism".
Outbreaks are raging around the globe with Covid-19 deaths nearing 2.2 million, and while wealthy countries fight over limited vaccine supplies, there are fears the less privileged will not get access for a long time.
British-Swedish firm AstraZeneca has said it can only deliver a fraction of its vaccine doses promised to the EU and Britain because of production problems, but both sides are demanding their pledges are met. — AFP
The EU's medicines regulator says the Pfizer/BioNTech coronavirus jab has no link to reported post-vaccination deaths and no new side effects, based on the first data from the vaccine's rollout.
The European Medicines Agency says it had looked at the deaths, including a number in the elderly, and "concluded that the data did not show a link to vaccination with Comirnaty (the vaccine) and the cases do not raise a safety concern." — AFP
US biotech firm Novavax says its two-shot COVID-19 vaccine showed an overall efficacy of 89.3% in a major Phase 3 clinical trial in Britain, and remained highly effective against a variant first identified there.
But the positive news was partly offset by other results that showed it offered significantly less protection against a highly transmissible variant of the coronavirus first identified in South Africa, which is spreading rapidly around the world.
Novavax says it began working on new vaccines against emerging strains in early January and expects to select ideal candid. — AFP
US biotech firm Novavax said Thursday its two-shot COVID-19 vaccine showed an overall efficacy of 89.3% in a major Phase 3 clinical trial in Britain, and remained highly effective against a variant first identified there.
But the positive news was partly offset by other results that showed it offered significantly less protection against a highly transmissible variant of the coronavirus first identified in South Africa, which is spreading rapidly around the world.
Novavax said it began working on new vaccines against emerging strains in early January and expects to select ideal candidates in the coming days, then begin clinical testing in the second quarter of the year.
"NVX-CoV2373 has the potential to play an important role in solving this global public health crisis," said the company's president and CEO Stanley Erck, using Novavax's name for the vaccine.
"We look forward to continuing to work with our partners, collaborators, investigators and regulators around the world to make the vaccine available as quickly as possible." — AFP
The vaccine made by Pfizer and BioNTech appears to retain its effectiveness against coronavirus mutations in worrying new variants that have emerged recently in Britain and South Africa, the firms say.
Several new variants -- each with a cluster of genetic mutations -- have sparked fears over an increase in infectiousness as well as suggestions that the virus could begin to elude immune response, whether from prior infection or a vaccine. — AFP
Pfizer and BioNTech, makers of a COVID-19 vaccine, say on Thursday that their product is effective against coronavirus variants that have emerged in Britain and South Africa.
In a statement, the two companies say the "small differences" detected in tests comparing the original virus and the recent versions "are unlikely to lead to a significant reduction in the effectiveness of the vaccine". — AFP
Pharmaceutical group AstraZeneca has pulled out of a meeting with EU representatives meant to take place Wednesday to get to the bottom of delays of its COVID-19 vaccine, an EU official tells AFP.
The sudden cancellation by the Anglo-Swedish firm marked a dramatic new turn in escalating tensions between it and the European Commission, which is demanding AstraZeneca fulfil its contract for the vaccine doses. — AFP
AstraZeneca's CEO insists that the company was not selling vaccines ordered by the European Union to other countries at a profit, after delayed orders sparked fury from EU leaders.
The British-Swedish drugs firm admitted last week that it would not meet its contractual delivery commitments to the EU because of "reduced yields" in its European supply chain.
That prompted European Health Commissioner Stella Kyriakides to announce that the EU plans to start tracking vaccine shipments exported to non-member countries -- a sign of growing distrust. — AFP
World Health Organization experts on Tuesday cautiously backed delaying second injections of the Moderna coronavirus vaccine in some situations, and insisted international travellers should not be prioritised for COVID-19 jabs.
The WHO's Strategic Advisory Group of Experts on Immunisation (SAGE) said it was best to adhere to the tested interval of 28 days between doses of the Moderna vaccine, but that in "exceptional circumstances" the doses could be spaced as far as 42 days apart. — AFP
Companies producing COVID-19 vaccines "must deliver," EU chief Ursula von der Leyen insists on Tuesday as tensions mounted between her European Commission and pharmaceutical firms that have delayed deliveries.
"Europe invested billions to help develop the world's first COVID-19 vaccines," she says in a live video address to an online-only version of the annual World Economic Forum usually held in Davos, Switzerland.
"And now, the companies must deliver. They must honour their obligations," she says. — AFP
Pharma giant AstraZeneca defends the efficacy of its COVID-19 vaccine after media reports said the German government had doubts about its effectiveness among those over 65.
The Handelsblatt economic daily reported Monday that Berlin had estimated the efficacy of the jab among over-65s was just 8%, citing sources. — AFP
France's Pasteur Institute says on Monday it was ending development of a COVID-19 vaccine with US pharmaceutical company Merck after clinical trial results proved disappointing.
The partners announced a tie-up last May and had been developing a jab based on an existing measles vaccine but it failed to prove its efficacy in Phase 1 clinical trials, the Paris-based institute says in a statement. — AFP
Pfizer announces that it will provide up to 40 million of its COVID-19 vaccine doses to poorer countries on a non-profit basis, through the globally-pooled Covax facility.
While dozens of the world's richer countries have begun their vaccination campaigns in a bid to curb the pandemic, coronavirus jabs have been few and far between in the world's poorer nations. — AFP
The Hungarian government says it had reached a deal to buy large quantities of Russia's Sputnik V coronavirus vaccine, even though it has not been approved by the European Union's medicines watchdog.
"Hungary has concluded with Russia an agreement to buy in three phases large quantities of the Sputnik V vaccine; the contract has been negotiated, and signed during the night," Hungarian Foreign Minister Peter Szijjarto says in a video statement on his Facebook page after meeting the Russian health minister in Moscow. — AFP
The Brazilian government says that a shipment of two million doses of the British AstraZeneca-Oxford COVID-19 vaccine was finally set to arrive in the country from India.
The delivery Friday would be a much-needed boost to Brazil's vaccination program.
"The two million doses of AstraZeneca should arrive in Brazil Friday in the late afternoon," the ministry of health says in a statement. — AFP
Japan aims to start vaccinating the general public against the coronavirus in May -- just two months before the postponed Olympics -- following targeted jabs for the most vulnerable, reports said Wednesday.
The Yomiuri Shimbun newspaper said the government is hoping the majority of adults will be vaccinated by July, when the Games are due to open.
The country has agreed with pharmaceutical firms to receive enough doses for all 126 million residents and is working to approve the Pfizer jab as the first to be used in Japan from next month. — AFP
Mexican authorities are investigating the theft of several coronavirus vaccines from a public hospital, the army said Tuesday, underscoring the challenges of distributing the shots across the crime-plagued country.
Mexico, which has one of the world's highest COVID-19 death tolls, has deployed the military to guard the vaccines and prevent them falling into criminals' hands.
The army said that the stolen vaccines were under the control of a public health institution in a hospital in central Morelos state whose security is overseen by a private company.
"This theft could have been a dishonest act of self-interest by a member of the hospital's vaccination team," it said in a statement. — AFP
Serbia becomes one of the first European countries to receive a Chinese-made COVID-19 vaccine on Saturday when one million doses of a jab produced by Sinopharm arrived at Belgrade airport.
President Aleksandar Vucic posted a picture of himself on Instagram, standing next to the plane carrying the vaccine.
"We are proud of our friendship with China," he was quoted as saying by Beta news agency, telling reporters that he hoped to be inoculated with the Sinopharm vaccine in six or seven days. — AFP
Both Pope Francis and his predecessor, former pope Benedict XVI, have received the coronavirus vaccine, the Vatican says on Thursday.
"I can confirm that as part of the Vatican City State vaccination programme to date, the first dose of the Covid-19 vaccine has been administered to Pope Francis and the Pope Emeritus," spokesman Matteo Bruni says. — AFP
President Joko Widodo receives the first COVID-19 vaccine in Indonesia. The shot, from China's Sinovac, kicks off the massive immunization campaign in the country dealing with one of most number of cases in the region.
Indonesia opted to secure millions of doses from the Chinese pharmaceutical company whose lack of transparency about its vaccine sparks concerns. While the Philippines ordered 25 million doses, Indonesia secured 125.5 million from Sinovac.
Indonesia is also expecting 50 million doses from Astrazeneca, 150 million from American biotech firm Novovax, 60 million from Sinopharm and 20 million from CanSino, making it the third territory in the world with the most number of confirmed doses after Europe and the United States. (Handout photo)

The Chinese-developed Coronavac COVID-19 vaccine has demonstrated a 50 percent efficacy following tests in Brazil, the organization in charge of its production in the South American country says.
Coronavac has been given to frontline health care workers in close contact with coronavirus patients.
The Butantan Institute repeated its claim from last week that the vaccine is 78% effective in preventing mild cases that needed treatment and showed 100% efficacy in staving off moderate to serious cases. — AFP
Switzerland gives the green light Tuesday to the Moderna vaccine against the new coronavirus — the second jab it has authorised for use, joining the vaccine produced by Pfizer-BioNTech.
The Swissmedic regulatory authority said the vaccine from US firm Moderna had shown a "high efficacy rate", and could be deployed immediately.
The neighbouring European Union gave its approval to the Moderna vaccine last Wednesday, with Britain doing likewise on Friday. — AFP
AstraZeneca and Oxford University have applied for authorisation for their coronavirus vaccine in the EU with a decision possible by January 29, the European Medicines Agency says on Tuesday.
The jab would be the third available for the 27-nation European Union after the Pfizer-BioNTech and Moderna drugs, as the bloc struggles to speed up the rollout.
"EMA has received an application for conditional marketing authorisation for a COVID-19 vaccine developed by AstraZeneca and Oxford University," the Amsterdam-based regulator says in a statement. — AFP
Zamboanga City Mayor Maria Isabelle Climaco-Salazar formally signed Monday the multilateral agreement with AstraZeneca for the purchase of 410,000 doses of vaccines worth P100 million.
Salazar said she signed the agreement after the city council in a special session last week gave her the authority to enter the agreement for the purchase of the vaccines with AstraZeneca through the national government.
Salazar said the initial acquisition of 410,000 vaccines is intended for the 205,000 residents as part of the city's vaccination program against coronavirus disease (COVID-19). — The STAR/Roel Pareño
Officials say the manufacturers of two COVID-19 vaccines developed by Chinese pharmaceutical firm Sinovac and Oxford-AstraZeneca filed the first applications Friday for regulatory approval in hard-hit Brazil.
Federal health regulator Anvisa now has 10 days to respond to the applications, though it said that could include asking the sponsors for more information.
One application was submitted by the Butantan Institute, a public health center in Sao Paulo that is working with Sinovac to test and produce its CoronaVac vaccine. — AFP
The European Medicines Agency says on Wednesday that it had given the green light for US firm Moderna's coronavirus vaccine, the second jab for the disease to be cleared for use in the EU.
"EMA has recommended granting a conditional marketing authorisation for COVID-19 Vaccine Moderna to prevent coronavirus disease 2019 in people from 18 years of age," the Amsterdam-based regulator says in a statement. — AFP
Prime Minister Justin Trudeau says in his first address of the new year that he was "frustrated" by the slow pace of COVID-19 inoculations across Canada, three weeks into a vaccination campaign.
The Canadian government has so far distributed 424,500 doses of Pfizer-BioNTech and Moderna vaccines -- the only ones authorized to date in Canada -- to its provinces and territories responsible for the vaccination program, according to official figures.
But only about 148,000 doses of vaccine have been administered so far, according to local media. — AFP
Russian President Vladimir Putin and German Chancellor Angela Merkel discussed the possibility of jointly producing coronavirus vaccines in a phone call, the Kremlin says Tuesday.
"Issues of cooperation in combating the coronavirus pandemic were discussed with an emphasis on the possible prospects for joint production of vaccines," the Kremlin says in a statement. — AFP
Under mounting pressure to speed up coronavirus vaccinations, Australian Prime Minister Scott Morrison on Tuesday says he would not take "unnecessary risks" and emulate Britain's emergency drug approval.
While vaccinations are already well underway in many countries, Australia's pharmaceutical authority is not expected to rule on candidate drugs for around another month, and is aiming to administer the first doses by the end of March.
Pressed about that seemingly sluggish timetable, Morrison — who early in the pandemic boasted Australia would be "at the front of the queue" for any vaccine — suggested virus-ravaged countries like Britain had been forced to take risks with emergency approvals. — AFP
Mexico authorizes the coronavirus vaccine developed by AstraZeneca and the University of Oxford for emergency use in the country, which has one of the world's highest COVID-19 death tolls.
Deputy health minister Hugo Lopez-Gatell announces on Twitter that Mexican regulators had approved the vaccine, which has also been authorized by Britain, India and Argentina.
It is the second coronavirus vaccine authorized by Mexico, which on December 24 began a mass immunization program using the Pfizer-BioNTech shot, with priority given to health workers. — AFP
Chinese drugmakers Sinopharm and Sinovac may seek emergency use authorization for their COVID-19 vaccines in the Philippines this week, according to Philippine Ambassador to China Chito Sta. Romana.
"We ask them when they will file for [emergency use authorization] with the [Food and Drug Administration] in the Philippines. Apparently, their plan right now, according to them is that they hope to file in the next few days sometime this week," Sta. Romana says.
Britain on Monday begins the mass rollout of the Oxford-AstraZeneca coronavirus vaccine, a cheap and easy to distribute shot that experts hope will help crush the pandemic.
Brian Pinker, an 82-year-old retired maintenance manager, received the jab at Oxford's Churchill Hospital, NHS England says. — AFP
A Brazilian association of private health clinics said Sunday it was negotiating with Indian pharmaceutical firm Bharat Biotech to buy five million doses of its COVID-19 vaccine, which India has just authorized for emergency use.
The Brazilian Association of Vaccine Clinics (ABCVAC) confirmed on its website it had signed a memorandum of understanding with the Indian firm to purchase its Covaxin vaccine, which is currently in the final stage of clinical trials.
Any final deal would be subject to approval by Brazil's health regulator, Anvisa, which has yet to approve any vaccines against the new coronavirus. — AFP
India has authorised the emergency use of two coronavirus vaccines developed by AstraZeneca and Oxford University and by local pharmaceutical firm Bharat Biotech, the country's drug regulator says Sunday.
"The... vaccines of Serum Institute (AstraZeneca/Oxford vaccine) and Bharat Biotech are being approved for restricted use in emergency situations," the Drugs Controller General of India, V.G. Somani, says at a briefing.
The approval is expected to kick off one of the world's biggest vaccination drives in coming days in the country of 1.3 billion people. — AFP
India stages nationwide drills to start one of the world's biggest coronavirus vaccination programs as the drug regulator prepared to approve the first vaccine.
A government panel on Friday recommended emergency use of the AstraZeneca-Oxford University shot and the first injections could be given in the coming week after the Drugs Control Authority of India gives final approval.
India, which has the world's second highest number of pandemic cases -- more than 10.2 million -- has set an ambitious target of inoculating 300 million of its 1.3 billion people by mid-2021. — AFP
A coronavirus vaccine developed by drug firm AstraZeneca and Oxford University has been approved for use in Britain, the government announced on Wednesday, paving the way for a mass rollout.
A government spokesman said it has accepted a recommendation from the Medicines and Healthcare products Regulatory Agency (MHRA) "to authorise Oxford University/AstraZeneca's Covid-19 vaccine for use", making Britain the first nation to approve the jab. — AFP
A group of doctors calls for safe and transparent use of vaccines for the coronavirus disease.
According to Health Professionals Alliance Against COVID-19, it is alarming that some Filipinos are receiving vaccines against the deadly illness that have not been registered with the Food and Drug Administration.
"The success of any vaccination program is hinged on mutual trust between the public and institutions," the group says in a statement.
Argentina has launched a COVID-19 vaccination campaign with the Sputnik V shots developed by Russia, the government says.
It is the first country in the Americas to use the vaccine against the pandemic.
The vaccination drive began simultaneously around the country with frontline healthcare workers given priority. — AFP
Belarus begins a vaccination drive against coronavirus using the Sputnik V jab, becoming the first country outside Russia to use the vaccine developed by Moscow.
Belarus, with a population of around 9.5 million people, has registered more than 188,000 cases of coronavirus infections and nearly 1,400 deaths.
"Today the first vaccine shipment has arrived in Belarus," says the Russian Direct Investment Fund (RDIF) which financed Sputnik V. — AFP
Hungary says it received 6,000 doses of Russia's controversial coronavirus vaccine on Monday, in a new display of Budapest's maverick vaccine policy.
"6,000 doses of Russian vaccine crossed Hungary's border with Slovakia," Hungarian Foreign Minister Peter Szijjarto says in a Facebook video message.
The consignment was taken to Budapest for a decision by Hungarian experts on how to use it, he said, without providing details on the vaccine's potential rollout. — AFP
Clinical trials to determine the safety and efficacy of a COVID-19 vaccine candidate from American biotech company Novavax have begun in the United States and Mexico, the US National Institutes of Health (NIH) announced Monday.
A similar Phase 3 trial for the same vaccine, called NVX-CoV2373, is also under way in the United Kingdom, where about 15,000 volunteers have been recruited.
In the US and Mexico, the new trials will include around 30,000 volunteers over the age of 18. — AFP
Lebanon will receive its first shipment of coronavirus vaccines in February from Pfizer-BioNTech, the health ministry said Sunday.
"Lebanon will receive the vaccine in mid-February in instalments," it said in a statement.
"It will cover 15 percent of the population."
The ministry said it would later secure "additional vaccines" to cover another 20 percent of the population as part of an agreement with COVAX, without specifying which brand.
COVAX is an international initiative that aims to ensure equitable access to coronavirus vaccines for all countries. — AFP
Several EU nations were set to begin vaccinating their most vulnerable groups Sunday as a new coronavirus variant spread internationally and the WHO warned the current pandemic will not be the last.
The first doses of the Pfizer-BioNTech jab arrived in hard-hit Italy, Spain and France on Saturday, ready for distribution to retirement homes and care staff.
The approval and roll-out of vaccines has boosted hopes that 2021 could bring a respite from the pandemic, which has killed more than 1.7 million people since emerging in China late last year.
However, in a video message ahead of the first International Day of Epidemic Preparedness, World Health Organization chief Tedros Adhanom Ghebreyesus said it was time to learn the lessons from the COVID-19 pandemic.
"History tells us that this will not be the last pandemic, and epidemics are a fact of life," said Tedros. — AFP
A lawmaker urges the Food and Drug Administration to issue a certificate of product registration for Pfizer and Moderna COVID-19 vaccines.
"Gamitin nilang batayan ang mga decision ng regulatory agencies ng US, UK, European Union, Singapore, Japan, at iba pang bansa na alam ng ating FDA na may mataas na kalidad na approval process," says Rep. Alfredo Garbin Jr. (AKO Bicol) in a statement.
Morocco says it had ordered 65 million doses of novel coronavirus vaccine, as the North African kingdom prepared to launch a vaccination campaign targeting 25 million people.
"Preparations have reached very advanced stages," Health Minister Khalid Ait Taleb says in a statement on the roll-out plans.
"Field exercises covering all stages of the process of vaccinating citizens have been put in place." — AFP
Turkey will receive its first shipment of China's Sinovac coronavirus vaccine within days as preliminary domestic tests showed it was 91% effective, Health Minister Fahrettin Koca says.
Ankara in the next few days will also sign a deal with Pfizer/BioNTech for 4.5 million doses, with the option to buy 30 million more from the US pharmaceutical giant and its German partner, Koca says. — AFP
Mexico on Wednesday became the first Latin American country to receive coronavirus vaccines for mass immunization against a disease that has had a devastating impact across much of the region.
The government plans to start inoculations on Thursday after the first 3,000 doses produced by US pharmaceutical giant Pfizer and its German partner BioNTech arrived by courier plane from Belgium.
The vaccines were whisked to a military installation in the south of Mexico City, guarded by a security escort to prevent them from falling into the hands of the country's powerful criminal gangs.
"Today is the beginning of the end of this pandemic," Foreign Minister Marcelo Ebrard told reporters at the airport. — AFP
Serbia will begin vaccinating people against COVID-19 on Thursday, President Aleksandar Vucic says Wednesday, making the Balkan state one of the first in Europe to launch a campaign with the Pfizer-BioNTech shot.
Officials say Serbia has received nearly 5,000 doses of the vaccine, which is already in use in the United States and Britain.
Meanwhile European Union countries plan to roll out the vaccine on December 27. — AFP
Switzerland starts its COVID-19 vaccine rollout on Wednesday, with a care home resident in her 90s becoming the first person in the country to receive the jab.
The woman, who lives in the Lucerne region in central Switzerland, was given the Pfizer-BioNTech vaccine, just four days after it was approved by national regulators.
"I am very satisfied that we have now been able to start vaccinations in the canton of Lucerne," the region's health services chief Guido Graf says in a statement. -- AFP
Mexico will begin COVID-19 immunizations on Thursday, a day after the country receives its first batch of Pfizer-BioNTech vaccines, Undersecretary of Health Hugo Lopez-Gatell says Tuesday.
"Tomorrow (Wednesday) the first consignment of the Pfizer vaccine against SARS-CoV-2 arrives," he says on Twitter.
"There will be a press opportunity and then the vaccine will be safeguarded until its use on Thursday, December 24, the day vaccinations start." — AFP
US President-elect Joe Biden received a Covid-19 vaccine live on television Monday in a campaign to boost Americans' confidence in the jabs -- and in marked contrast to President Donald Trump's mixed messaging.
The 78-year-old incoming president got the Pfizer vaccine at the Christiana Hospital in Newark, Delaware. His wife Jill received the shot earlier, the presidential transition team said.
Biden told Americans "there's nothing to worry about" when they get vaccinated and that in the meantime they should keep wearing masks and "listen to the experts." — AFP
Interpol chief Juergen Stock predicts Monday a sharp rise in crimes with robbers seeking to get their hands on precious vaccines aimed at stopping the coronavirus pandemic.
"With vaccines rolling out, crime will increase dramatically," Stock tells business weekly WirtschaftsWoche. "We will see thefts and warehouse break-ins and attacks on vaccine shipments." — AFP
China plans to start opening its vaccination program to members of the public in southwestern Sichuan province early next year, health officials says, despite the inoculations not yet receiving official approval.
At least one million people have already received a jab in China after vaccine candidates were approved for "emergency use", but so far they have been limited to priority groups such as state employees and international students. — AFP
A US panel of experts votes to recommend emergency approval of Moderna's COVID-19 vaccine, paving the way for six million doses to start shipping as soon as this weekend.
The Food and Drug Administration is now expected to imminently grant an emergency use authorization, which would make Moderna's vaccine the second to be approved in a Western country. — AFP
President Vladimir Putin says he will receive the Russian-developed Sputnik V coronavirus jab once it is approved for people his age, praising the vaccine as safe and effective.
The 68-year-old Russian president says at his annual end-of-year press conference that he had not been vaccinated yet, but "will definitely do so as soon as it becomes possible" according to expert advice. — AFP
US experts meet to decide whether to recommend approval of Moderna's COVID-19 vaccine, potentially paving the way for rollout early next week.
The meeting comes after the Pfizer-BioNTech vaccine received an emergency use authorization and the first three million doses were distributed in the world's worst-hit country this week.
Thursday's meeting will be live-streamed to the public, and will end with a vote by the two dozen independent scientists and industry representatives. — AFP
Indonesian President Joko Widodo says Wednesday he would be the first person in the country to be vaccinated for COVID-19 as he unveiled a campaign promising free inoculations for everyone in world's fourth most-populous nation.
Wikodo's announcement comes as Indonesia battles misinformation over the virus in order to stave off a fresh wave of infections, with some 630,000 recorded by Wednesday and more than 19,000 deaths. — AFP
The EU's 27 member countries aim to start COVID-19 vaccinations on "the same day" in a sign of unity, European Commission chief Ursula von der Leyen says.
Her statement to the European Parliament came as pressure mounted on the bloc to catch up with the United States and Britain, which have already started inoculating people with a vaccine made by Pfizer and BioNTech. — AFP
A Chinese pharmaceutical company says it had agreed to buy at least 100 million doses of the coronavirus vaccine from German company BioNTech, subject to Beijing approving its use.
China has been rapidly developing its own COVID-19 vaccine candidates and ramping up production facilities, but local firms have also been partnering with foreign developers to supply the world's most populous country.
Shanghai Fosun Pharmaceutical Group says its subsidiary had entered into an agreement with the German firm aimed at ensuring "an adequate supply" of vaccines in China, adding it will make an initial payment of 125 million euros ($152 million) before year-end for 50 million doses. — AFP
Sen. Risa Hontiveros on Wednesday urged the government to avoid political agendas in the procurement of COVID-19 vaccines amid concerns raised over the plan to buy 25 million doses from Chinese pharmaceutical Sinovac.
"Let's make it clear: The vaccine is a health solution, not a political favor. We should buy vaccines to protect our people from COVID-19. Not to advance anyone’s political agenda," she says.
Sinovac is among the pharma companies applying for approval to hold clinical trials for the vaccine in the Philippines.
At least a fifth of the world's population may not have access to a Covid-19 vaccine until 2022, according to a study published Wednesday, with wealthier nations reserving more than half of next year's potential doses.
With hopes that vaccines can bring an end to a pandemic that has killed some 1.6 million people, countries including the United States, Britain and the United Arab Emirates have already begun rolling out immunisation programmes.
Eager to increase their chances of having access to at least one of the dozens of vaccines in development, many nations have snapped up allocations of several different drugs. — AFP
Germany says Tuesday it wants the European Union to approve the BioNTech/Pfizer COVID-19 vaccine "before Christmas", as criticism grows of the EU health regulator's plan to make a decision by December 29 at the latest.
"The goal is to get approval before Christmas," German Health Minister Jens Spahn tells reporters. "We want to start vaccinating in Germany before the end of the year."
Britain and the United States have already approved the vaccine and started vaccinating their citizens. — AFP
Jordan announces that it had approved emergency use of the Pfizer-BioNTech coronavirus vaccine, as the United States kicked off a mass vaccination drive.
The Jordanian Food and Drug Administration did not specify when it would begin the rollout of the vaccine by US pharmaceuticals giant Pfizer and its German partner BioNTech. — AFP
The head of the UN children's agency, UNICEF, calls for teachers to be among those given priority access to the COVID-19 vaccines.
"The COVID-19 pandemic has wreaked havoc on children's education around the globe. Vaccinating teachers is a critical step towards putting it back on track," UNICEF chief Henrietta Fore says in a statement.
Teachers should be "prioritized to receive the Covid-19 vaccine, once frontline health personnel and high-risk populations are vaccinated," she says. — AFP
Facing record levels of coronavirus, the United States began shipping vaccine nationwide on Sunday as it launched a massive immunization effort, while in Germany an explosion of cases forced a return to partial lockdown.
Delivery trucks with special refrigeration equipment rolled out of a facility in Kalamazoo, Michigan, as part of a public-private plan to ship millions of doses of the newly approved Pfizer-BioNtech vaccine to vulnerable Americans.
Delivery services FedEx and UPS are deploying fleets of trucks and planes to carry their precious cargo -- sometimes under armed guard — to all 50 states, where healthcare workers and nursing-home residents will be first in line.
As the historical mobilization unfolds, an initial 2.9 million doses are to be delivered by Wednesday, with officials saying 20 million Americans could receive the two-shot regimen by year end, and 100 million by March. —AFP
Americans will start receiving the Pfizer-BioNTech COVID-19 vaccine on Monday, the official in charge of the distribution operation says.
General Gus Perna tells reporters the first doses will be shipped Sunday and that starting Monday morning, "We are operationally 100% confident that we will get the vaccines to the American people."
"Expect 145 sites across all the states to receive vaccine on Monday, another 425 sites on Tuesday. And the final 66 sites on Wednesday, which will complete the initial delivery of the Pfizer-BioNTech vaccine," he says. — AFP
Peru temporarily suspends clinical trials of a COVID-19 vaccine made by Chinese drug giant Sinopharm after detecting neurological problems in one of its test volunteers.
The National Institute of Health says that it had decided to interrupt the trial after a volunteer had difficulty moving their arms, according to local media.
"Several days ago we signaled, as we are required, to the regulatory authorities that one of our participants (in trials) presented neurological symptoms which could correspond to a condition called Guillain-Barre syndrome," says chief researcher German Malaga in comments to the press. — AFP
The US says it was purchasing 100 million more doses of the COVID-19 vaccine candidate developed by Moderna, amid reports the government passed on the opportunity to secure more supply of the Pfizer jab.
The agreement brings the total number of Moderna doses bought by the US to 200 million, enough to immunize 100 million people with the two-shot regimen. — AFP
US issues emergency approval to Pfizer-BioNTech Covid vaccine, according to statement.
A health official says Mexico's health regulator granted emergency authorization to the Pfizer-BioNTech coronavirus vaccine.
"Mexico is the fourth country whose health regulatory agency, Cofepris, has granted emergency use authorization to the Pfizer-BioNTech vaccine," says Health Undersecretary Hugo Lopez-Gatell. — AFP
France's Sanofi and Britain's GSK said Friday their COVID-19 vaccines will not be ready until the end of 2021, after interim results showed a low immune response in older adults.
"Sanofi and GSK announce a delay in their adjuvanted recombinant protein-based COVID-19 vaccine programme to improve immune response in older adults," a statement said, adding that the vaccine's potential availability had been pushed back "from mid-2021 to Q4 2021". — AFP
Indonesia is betting that China-made coronavirus vaccines can help it tackle one of the worst outbreaks in Asia, but analysts warn it is a wager that could leave them holding a high-interest diplomatic IOU.
Beijing has promised poorer nations priority access to its inoculations, in an attempt to repair an image tarnished by the pandemic, which started in the central Chinese city of Wuhan.
This week, Indonesia received 1.2 million doses of a COVID-19 vaccine made by China's Sinovac, with another 1.8 million set to arrive next month, but experts say this access could have strings attached. — AFP
The United Arab Emirates on Wednesday officially registers the coronavirus vaccine produced by Chinese drug giant Sinopharm, saying it was 86% effective according to analysis of third-phase trials.
The health ministry "has announced the official registration" of the vaccine, state news agency WAM says, without elaborating on how it would now be used.
The vaccine has been undergoing third-phase trials in the Emirates since July, and it was approved for emergency use for healthcare workers in September. — AFP
Britain's Prime Minister Boris Johnson calls the roll-out of a new Pfizer-BioNTech vaccine against COVID-19 a "tremendous shot in the arm for the entire nation".
But with most people not expected to get vaccinated until early 2021, he says the public still needed to be careful to stop the spread of the virus.
"We can't afford to relax," he says on a visit to a central London hospital. — AFP
Briton, 90, first person to receive Pfizer's approved COVID-19 vaccine, according to BBC.
Britain hails a turning point in the fight against the coronavirus pandemic, as it begins the biggest vaccination programme in the country's history with a new COVID-19 jab.
The first patients in line on what has been dubbed "V-Day" -- the over-80s, care home workers and at-risk frontline health and social care staff -- will roll up their sleeves for an initial dose from early morning.
They will then require a second jab in 21 days' time. — AFP
Officials say Indonesia has received 1.2 million doses of a COVID-19 vaccine made by China's Sinovac as the world's fourth most populous nation struggles to get soaring case rates under control.
The doses arrived in Jakarta late Sunday on a flight from Beijing, with another 1.8 million expected to be sent again next month.
Although Chinese regulators have yet to clear any of country's vaccines for mass distribution, they have approved some advanced candidates for emergency use. — AFP
Trucks and cargo planes are at the ready to distribute millions of doses of coronavirus vaccine across the United States, a complex task led by a four-star general that will ultimately proceed more slowly than initially expected.
US Army General Gus Perna, in charge of logistics for the government's Operation Warp Speed, has been putting his troops -- a mix of soldiers and health experts -- through dry runs for weeks, in anticipation of the day when a vaccine is approved.
The US Food and Drug Administration is due to grant emergency use approval to the vaccines made by Pfizer-BioNTech and Moderna, likely soon after December 10 and 17, respectively. — AFP
Britain on Monday prepares to start its biggest ever immunisation campaign but health officials warned the drive to inoculate millions against COVID-19 would be a "marathon" stretching well into next year.
The world-first rollout of the Pfizer-BioNTech vaccine is due to start on Tuesday — dubbed "V-Day" by Health Secretary Matt Hancock, who has volunteered to take it live on television to assuage any public doubts over the rapid approval.
Croydon University Hospital in south London is one of 50 clinical hubs that started receiving the country's initial consignment of 800,000 doses over the weekend, from a Pfizer plant in Belgium. — AFP
Chinese pharmaceutical firm Sinovac Biotech has secured half a billion dollars in extra funding to produce its COVID-19 vaccine, it says Monday, as the country races to roll out a jab for general use.
Beijing has largely brought the virus under control, with only 281 active cases still receiving treatment, according to official figures.
But it has promised to make its vaccines available as a "global public good" as it seeks to counter global criticism for its early handling of the pandemic. — AFP
The World Health Organization said it was considering introducing electronic vaccination certificates, as hopes for an end to the pandemic were boosted after Britain became the first country to approve use of a Covid-19 vaccine.
"We are looking very closely into the use of technology in this Covid-19 response and one of them is how can we work with members states towards something called an e-vaccination certificate," WHO Europe expert Siddhartha Datta told an online press briefing Thursday.
Introducing such a certificate, which would make it possible to identify and monitor people who have been vaccinated, has not been finalised and would have to be drawn up in accordance with national laws, Datta said.
It would not be an immunity passport, which is supposed to assure that its carrier is protected against the disease because they have been infected and recovered. — AFP
Moderna plans to have 100 to 125 million doses of its COVID-19 vaccine available in the first quarter of 2021, the vast majority of which will go to the United States, the biotechnology company announces.
Between 85 and 100 million of the doses will be distributed in the United States, with the rest of the world receiving the remaining 15 to 25 million, the Cambridge, Massachusetts-based company says in a statement.
Moderna also confirms that it expects to have 20 million vaccine doses available in the US by the end of 2020. — AFP
Britain's approval of BioNTech-Pfizer's vaccine against COVID-19 marks a "historic moment" in the battle against the pandemic, the US pharma group's chief executive says Wednesday, after his company won the first such authorisation in the West.
"Today's Emergency Use Authorisation in the UK marks a historic moment in the fight against COVID-19," says Pfizer CEO Albert Bourla.
The US company and Germany's BioNTech adds that they expected further regulatory decisions from other countries "in the coming days and weeks". — AFP
UK approves Pfizer-BioNTech vaccine for rollout from "next week".
Britain on Wednesday becomes the first country to approve Pfizer-BioNTech's COVID-19 vaccine for general use and says it would be introduced next week.
"The government has today accepted the recommendation from the independent Medicines and Healthcare products Regulatory Agency (MHRA) to approve Pfizer-BioNTech's Covid-19 vaccine for use," the department of health says in a statement.
"The vaccine will be made available across the UK from next week," the statement says, with priority groups including care home residents, health and care staff. — AFP
President Rodrigo Duterte grants authority to the Food and Drug Administration to issue emergency use authorization for COVID-19 vaccines, Executive Secretary Salvador Medialdea says.
Duterte's Executive Order 121 also allows FDA to release emergency use authorization for COVID-19 drugs, prescribing conditions and for other purposes.
The Pfizer and Moderna COVID-19 vaccines could be approved in a matter of weeks, but who in the United States will get them first?
A high level panel of US experts on Tuesday voted that health care workers and residents of long-term care facilities should be prioritized in the first phase.
"I believe that my vote reflects maximum benefits, minimum harm, promoting justice and mitigating the health inequalities that exist, with regard to distribution of this vaccine," said Jose Romero, chair of the Advisory Committee on Immunization Practices at the Centers for Disease Control and Prevention.
As the sequence continues though, US experts may differ from other countries in prioritizing "critical workers" who keep society running — potentially even before people at highest risk. — AFP
The European Medicines Agency (EMA) on Tuesday says it would hold an extraordinary meeting on December 29 "at the latest" to consider emergency approval for a COVID-19 vaccine developed by Germany's BioNTech and US giant Pfizer.
"If the data submitted are robust enough to conclude on the quality, safety and effectiveness of the vaccine, EMA... will conclude its assessment during an extraordinary meeting scheduled for 29 December at the latest," the EU regulator says in a statement.
Pfizer and BioNTech earlier announced they had formally applied for conditional EU approval for their jab, following a similar request in the United States. — AFP
Germany's BioNTech and its US partner Pfizer say they had applied for EU regulatory approval for their COVID-19 vaccine, raising hopes that the first jabs could be administered in December.
The two companies say in a statement that they had submitted an application to the European Medicines Agency on Monday "for Conditional Marketing Authorisation (CMA)" for their vaccine, after tests showed it was 95 percent effective against the novel coronavirus.
If approved, the jab could potentially be rolled out "in Europe before the end of 2020", the statement says. — AFP
US President Donald Trump's controversial coronavirus adviser resigned Monday, while hopes for a first wave of vaccinations before the end of 2020 received a further boost with an announcement from US firm Moderna.
Scott Atlas, a favored coronavirus adviser of the US president, who tweeted in October "Masks work? NO," has submitted his resignation, effective as of December 1, Fox News reported.
Lacking relevant experience or qualifications in public health or infectious disease, he also called in November for people in Michigan to "rise up" against COVID-19 measures. — AFP
A think tank questions the rush to sign a vaccine supply deal with British-Swedish drugmaker AstraZeneca as experts doubt its reported success rate.
“With the new global trials involving the low-dose regimen, it is premature for government to make a supply commitment to AstraZeneca, unless and until there is a showing that the new trials will yield the same ninety-percent efficacy rating in its limited trial run,” says Terry Ridon, Infrawatch PH convenor.

As a string of Covid-19 vaccines near approval, Frankfurt Airport staff are gearing up to handle the unprecedented logistical challenge of transporting millions of life-saving doses worldwide.
Frankfurt is Europe's largest hub for transporting pharmaceutical goods, and will be key to the success of inoculating millions of people against the deadly coronavirus. — AFP
One of the developers of Russia's Sputnik V coronavirus vaccine announces that India-based drugmaker Hetero will produce over 100 million doses of the jab.
"Hetero, one of India's leading generic pharmaceutical companies, have agreed to produce in India over 100 million doses per year of the world's first registered vaccine against the novel coronavirus infection -– Sputnik V," the Russian Direct Investment Fund says in a statement, adding that production was expected to start in early 2021.
Earlier this week, Russia said interim results from the Sputnik V clinical trails showed the vaccine was 95% effective, similar to other international vaccine makers that have also published test results showing efficacy rates of 90% and higher. — AFP
The World Health Organization urges African countries to improve their capacity to vaccinate populations against COVID-19, warning the continent was still "far from ready" for mass immunisation.
With three coronavirus vaccines now showing efficacy rates of 70% or more, the UN body called on Africa to "ramp up" preparations for "the continent's largest ever immunisation drive".
The African region is so far only 33 percent ready to roll out Covid-19 vaccines, the World Health Organization says in a statement. — AFP
The British government says it has asked its independent medicines regulator to assess AstraZeneca's coronavirus vaccine as part of the formal approval process for the drug to be rolled out by the end of the year.
More than 1.4 million people have died since the novel coronavirus emerged in China late last year, and three drug developers -- Pfizer/BioNTech, Moderna and AstraZeneca/Oxford University -- are currently applying for approval for their vaccines to be used as early as December.
AstraZeneca has completed Phase III clinical trials of its vaccine, the last stage before regulatory approval. — AFP
Cash-strapped Latin American governments face severe geographical, economic and social challenges in trying to ensure life-saving COVID-19 vaccines are made available to vulnerable populations, experts say.
Megacities like Sao Paulo, mountain ranges like the Andes as well as the vast Amazon basin pose just a few of the geographical problems for distributors, given the vital need to maintain the cold chain to preserve the vaccines.
Transporting vaccines "to the most distant parts of the big cities and to peripheral neighborhoods, with the need to conserve the cold chain, will be the first major challenge," Colombian epidemiologist Carlos Trillos told AFP.
Governments also face a race against time to provide training to those handling the doses throughout the cold chain, he said. — AFP
The United States plans to distribute 6.4 million doses of the Pfizer-BioNTech COVID-19 vaccine in the first week after it is cleared for emergency use, which is likely next month, officials say Tuesday.
A committee of the Food and Drug Administration meets on December 10 to decide whether to green light the medicine, with the US confronted by soaring numbers of deaths and new cases.
Latest figures on Tuesday showed that the country had recorded a total of 259,600 COVID deaths and 12.5 million cases — with over 2,000 deaths and 167,000 new cases in just the last 24 hours.
General Gustave Perna, chief operations officer for the government's Operation Warp Speed, told reporters some 40 million doses of vaccine would be available by the end of December.
That figure includes another vaccine developed by Moderna and the National Institutes for Health, which announced some preliminary efficacy results last week and is also close to applying for emergency approval. — AFP
The United States plans to distribute 6.4 million doses of the Pfizer-BioNTech COVID-19 vaccine in the first week after it is cleared for emergency use, which is likely next month, officials say.
A committee of the Food and Drug Administration meets on December 10 to decide whether to green light the medicine, with the US confronted by soaring numbers of deaths and new cases. — AFP
Russia's Sputnik V coronavirus vaccine is 95% effective according to a second interim analysis of clinical trial data, its developers said on Tuesday.
The calculations were based on preliminary data obtained 42 days after the first dose, Russia's health ministry, the state-run Gamaleya research centre and the Russian Direct Investment Fund (RDIF) said in a statement.
They did not note the number of cases used to make the calculation, however. — AFP
European stock markets opened firmer Monday, buoyed as positive news on another coronavirus vaccine trial helped offset concerns over soaring case numbers, dealers said.
In London, the FTSE 100 index of leading shares was up 0.5 percent at 6,382.36 points.
In the Eurozone, the Paris CAC 40 gained nearly one percent to 5,543.83 points and the Frankfurt DAX put on 0.6 percent to 13,219.25 points.
Shortly before the markets opened, British drugs group AstraZeneca and the University of Oxford said their jointly-developed vaccine against COVID-19 has shown "an average efficacy of 70 percent" in trials, and up to 90 percent in one dosage combination. — AFP
The United States hopes to begin coronavirus vaccinations in early December, a top government health official said Sunday, the latest positive news to emerge even as cases surge across the worst-hit nation and elsewhere around the globe.
The beginning of vaccinations could be a crucial shift in the battle against a virus that has claimed more than 1.4 million lives worldwide, including 255,000 just in the US, since emerging from China late last year.
Encouraging results from vaccine trials have bolstered hopes for an end to the pandemic, as nations reimpose restrictions and lockdowns that slowed the spread earlier this year but turned lives and economies upside down across the globe. — AFP
A leading COVID-19 vaccine candidate has shown to safely produce a robust immune response in healthy older adults, its British makers said Thursday as it released its phase 2 trial results.
The vaccine, developed by the University of Oxford and AstraZeneca, produced fewer side effects in people aged 56 and over than in younger people — a significant finding given that COVID-19 disproportionately causes severe illness among seniors.
The manufacturers said the vaccine was undergoing larger, more comprehensive phase 3 trials to confirm the results. — AFP
The biotech company Pfizer says Wednesday that a completed study of its experimental COVID-19 vaccine showed it is 95% effective.
Pfizer says the vaccine had no serious side effects and that the company will apply for emergency use authorization from US regulators within a matter of days.
"The study results mark an important step in this historic eight-month journey to bring forward a vaccine capable of helping to end this devastating pandemic," says Pfizer CEO Albert Bourla. — AFP
Pfizer is "very close" to applying for an emergency use approval for its COVID-19 vaccine after collecting safety data to submit to US regulators, the company's CEO said Tuesday, according to a report.
The pharmaceutical giant announced last week preliminary results from a late-stage clinical trial showing the injections it had co-developed with Germany's BioNTech was more than 90 percent effective after the second dose.
"We are very close to submitting for an emergency use authorization," Albert Bourla says. "We will announce it as soon as we are doing it."
— AFP
The United States' top infectious disease scientist on Monday hailed early results from Moderna's Covid-19 vaccine as "stunningly impressive."
"The idea that we have a 94.5 percent" effective vaccine is stunningly impressive," he told AFP.
"It is really a spectacular result that I don't think anybody had anticipated it would be this good." — AFP
Global hopes of vanquishing the coronavirus pandemic were boosted Monday after a second vaccine was found to be nearly 95 percent effective in a trial, bringing much-needed optimism amid surging infections and grueling new restrictions.
The news from the US biotech firm Moderna comes after similar results were announced last week for a vaccine candidate developed by pharma giant Pfizer and its German partner BioNTech.
Major stock markets surged Monday in response, building on a boom sparked by the Pfizer news one week ago.
Moderna, whose results stem from a clinical trial of more than 30,000 participants, expects to have approximately 20 million doses ready to ship in the United States by year-end. — AFP
Moderna on Monday announces its experimental vaccine against COVID-19 was shown to be 94.5 percent effective according to early results from a clinical trial with more than 30,000 participants.
"This positive interim analysis from our Phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease," says Stephane Bancel, Moderna's CEO. — AFP
A sudden coronavirus cluster emerged in the Australian city of Adelaide on Monday after seven months without a significant outbreak there, with the virus again escaping from the country's hotel quarantine system.
South Australia state reported four cases had been detected in the city on Sunday, before the cluster grew sharply overnight to 17 people Monday — the largest there since April.
All but two of the 17 were members of the same large family, including one who was working in a hotel used to quarantine travellers returning from overseas. — AFP
Venezuelan President Nicolas Maduro said Sunday that his government has agreed to buy 10 million doses of Russia's Sputnik V vaccine against COVID-19.
The vaccines will come in the first quarter of 2021, Maduro said at an event in Caracas broadcast on government television, adding that "Venezuela will manufacture the Russian vaccine in the Venezuelan laboratories."
In August, Russia became the first country to register a vaccine against COVID-19, which it named Sputnik V after the world's first satellite launched into space in 1957.
However, the announcement has been met with skepticism in the international community. — AFP
Brazil's health regulator says it had suspended clinical trials of a Chinese-developed COVID-19 vaccine after an "adverse incident" involving a volunteer recipient, a blow for one of the most advanced vaccine candidates.
The setback for CoronaVac, developed by Chinese pharmaceutical firm Sinovac Biotech, came on the same day US pharmaceutical giant Pfizer said its own vaccine candidate had shown 90 percent effectiveness, sending global markets soaring and raising hopes of an end to the pandemic.
The Brazilian regulator, Anvisa, said in a statement it had "ruled to interrupt the clinical study of the CoronaVac vaccine after a serious adverse incident" on October 29. — AFP
The US Food and Drug Administration on Monday granted emergency approval to a synthetic antibody treatment against Covid-19 developed by Eli Lilly, after the drug was shown to reduce the risk of hospitalization and emergency room visits.
Bamlanivimab at a dose of 700 milligrams was authorized for the treatment of mild-to-moderate Covid-19 in adults and children aged 12 years and older who are at high risk for progressing to the severe form of the disease.
It is the first major drug to be approved that was designed specifically against the new coronavirus.
"As illustrated by today's action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments," said the agency's chief Stephen Hahn. — AFP
Brazil's health regulator says Monday it has suspended clinical trials of a Chinese-developed COVID-19 vaccine after an "adverse incident" involving a volunteer recipient, a blow for one of the most advanced vaccine candidates.
The regulator, Anvisa, says in a statement that it has "ruled to interrupt the clinical trial of the CoronaVac vaccine after a serious adverse incident" on October 29.
It says it could not give details on what happened because of privacy regulations, but that such incidents included death, potentially fatal side effects, serious disability, hospitalization, birth defects and other "clinically significant events." — AFP
US President-elect Joe Biden Monday hails as a cause for "hope" the news that a COVID-19 vaccine developed by Pfizer and BioNTech was 90 percent effective — but warned of a long battle still ahead.
"I congratulate the brilliant women and men who helped produce this breakthrough and to give us such cause for hope," Biden says in a statement, adding that he received advance notice of the announcement on Sunday night.
"At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away," he adds — stressing the continued importance of mask-wearing for the foreseeable future. — AFP
US President Donald Trump hails the "great news" Monday that a vaccine jointly developed by Pfizer and BioNTech was 90 percent effective in preventing COVID-19 infections.
"STOCK MARKET UP BIG, VACCINE COMING SOON. REPORT 90% EFFECTIVE. SUCH GREAT NEWS!" the president tweets minutes after Pfizer announced the development and days after he lost the US presidential election to Democrat Joe Biden.
His defeat was blamed, in part, on his administration's response to the coronavirus pandemic, with infections surging across the US in recent days. — AFP
Dubai ruler Sheikh Mohammed bin Rashid Al-Maktoum says he had received an experimental coronavirus vaccine, becoming the latest United Arab Emirates official to take part in the trials.
Two vaccines are undergoing third-phase trials in the UAE, one produced by Chinese drug giant Sinopharm, and Russia's Sputnik-V, named after the Soviet-era satellite.
"While receiving the COVID-19 vaccine today," Sheikh Mohammed captioned a photograph of himself he posted on Twitter with his sleeve rolled up, as a healthcare worker in full protective equipment administered the injection. — AFP
Brazil is a top testing ground for vaccines against COVID-19, but its plans for vaccinating its own population have been plunged into chaos by a political war waged by President Jair Bolsonaro.
Hit hard by the new coronavirus, Brazil has been tapped to help test several of the leading vaccine candidates, giving it a potential edge in the race to secure access to an eventual shot.
That could be a welcome silver lining for the country of 212 million people, which has the second-highest COVID-19 death toll in the world, at more than 157,000.
But one promising test vaccine, developed by Chinese pharmaceutical firm Sinovac Biotech, has triggered the ire of the far-right president, who last week canceled his health minister's plan to buy 46 million doses.
The vaccine's most visible proponent in Brazil is the governor of the large and wealthy state of Sao Paulo, Joao Doria, who also happens to be one of Bolsonaro's top opponents. — AFP
Brazil's health minister says the country would add the Chinese-made CoronaVac vaccine against COVID-19 to its national immunization program, despite a political and diplomatic row over whether to use it.
Health Minister Eduardo Pazuello says the federal government had reached a deal with Sao Paulo state, which is helping test and produce the vaccine, to buy 46 million doses to be administered starting in January.
"This vaccine will be Brazil's vaccine," in addition to another developed by Oxford University and pharmaceutical firm AstraZeneca, Pazuello says in a video meeting of the South American country's 27 governors. — AFP
The US Food and Drug Administration made public its guidance for issuing emergency approval for a COVID-19 vaccine on Tuesday, making it clear it wants to see follow-up two months after trial volunteers have their second dose.
It is therefore unlikely for President Donald Trump's administration to have a vaccine on the market before the November 3 election, something the president frequently says is on the cards.
"Data from Phase 3 studies should include a median follow-up duration of at least two months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine's benefit-risk profile," the document said. — AFP
The US Food and Drug Administration made public its guidance for issuing emergency approval for a COVID-19 vaccine on Tuesday, making it clear it wants to see follow-up two months after trial volunteers have their second dose.
It is therefore unlikely for US President Donald Trump's administration to have a vaccine on the market before the November 3 election, something the president frequently says is on the cards.
"Data from Phase 3 studies should include a median follow-up duration of at least two months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine's benefit-risk profile," the document said.
The two companies that are furthest along in their vaccine trials, Moderna and Pfizer, both began their final stages at the end of July, and both require two separate injections 28 days apart. — AFP
The World Bank says it has asked its board of directors to approve $12 billion to help poor countries purchase and distribute eventual vaccines against COVID-19.
The bank has already implemented emergency response programs in 111 countries and the extra money, if approved, would be aimed at low- and middle-income countries.
"An effective and safe COVID-19 vaccine is the most promising path forward for the world to reopen safely," a World Bank spokesman says. — AFP
Latin American leaders have appealed at the United Nations for free access to a future COVID-19 vaccine, urging major powers to share their know-how for the sake of global well-being.
Latin America has taken a heavy blow from Covid-19 with nearly nine million cases and more than 330,000 deaths, one third of the global total, according to an AFP tally based on official data.
"With the pandemic, as with poverty, nobody will be saved on their own," Argentine President Alberto Fernandez tells the UN General Assembly, which is taking place virtually due to the health crisis. — AFP
Science Secretary Fortunato dela Peña says the Philippines had signed six confidentiality agreements with foreign pharmaceutical companies for COVID-19 vaccine.
These are:
Russia's Sputnik V (clinical trial and local manufacturing)
China's Sinovac (clinical trial and local manufacturing)
China's Sinopharm (procurement)
China's ZFSW (clinical trial)
Australia's University of Queensland (clinical trial)
Taiwan's Adimmune (clinical trial)
More than 60 wealthy nations have joined a WHO-backed programme to facilitate poor countries' access to coronavirus vaccines, but the US and China are not on the list published Monday.
The World Health Organization has in coordination with the global vaccine alliance group Gavi and the Coalition for Epidemic Preparedness Innovations (CEPI) created a mechanism aimed at ensuring a more equitable distribution of any future COVID-19 vaccines.
But the mechanism, known as Covax, has struggled to raise the funds needed to provide for the 92 low-income countries and other economies that quickly signed up.
— AFP
President Donald Trump says that a coronavirus vaccine may be available within a month -- an acceleration of even his own surprisingly optimistic predictions -- but added that the pandemic could go away by itself.
"We're very close to having a vaccine," he tells a town hall question-and-answer session with voters in Pennsylvania aired on ABC News.
"We're within weeks of getting it you know -- could be three weeks, four weeks," he says. — AFP
A China-developed coronavirus vaccine could be ready for the public as early as November, a Chinese official has told state television, as the global race to clear the final round of trials heats up.
Chinese manufacturers have been bullish about development, with companies Sinovac Biotech and Sinopharm even putting their vaccine candidates on display at a trade fair in Beijing this month.
Representatives of the firms told AFP that they hope their vaccines will be approved after phase 3 trials as early as year-end.
And on late Monday, the chief biosafety expert at the Chinese Centre for Disease Control told state broadcaster CCTV that a vaccine would be available to the general public "around November or December."
Wu Guizhen did not specify which vaccine she was referring to, but said "based on the phase 3 clinical results, the current progress is very smooth." — AFP
Drugs giant AstraZeneca says a COVID-19 vaccine could still be available by as early as the end of this year, despite a randomized clinical trial in the UK being paused.
"We could still have a vaccine by the end of this year, early next year," the company's chief executive Pascal Soriot says, adding it depended on how fast regulators moved. — AFP
EU reserves 200 million more coronavirus vaccines from Pfizer.
Pharmaceutical company AstraZeneca says it has "voluntarily paused" a randomized clinical trial of its coronavirus vaccine in what it called a routine action after a volunteer developed an unexplained illness.
"As part of the ongoing randomized, controlled global trials of the Oxford coronavirus vaccine, our standard review process was triggered and we voluntarily paused vaccination to allow review of safety data by an independent committee," a spokesperson says in a statement. — AFP
Saudi King Salman and Russian President Vladimir Putin discussed Monday the possible joint production of a Russian coronavirus vaccine, the Kremlin said.
In early August, Russia said it had developed the world's first vaccine against the virus and claimed that more than a billion doses had been pre-ordered by 20 countries, including Saudi Arabia.
Russia's sovereign wealth fund has provided much of the financing, and it has identified Saudi Arabia as one of the countries interested in the vaccine.
On Monday, Putin and Salman discussed "collective efforts aimed at overcoming the negative impact of the coronavirus pandemic," a Kremlin statement said, while noting the call had come at the Saudi king's initiative. — AFP
China has put its homegrown coronavirus vaccines on display for the first time, as the country where the contagion was discovered looks to shape the narrative surrounding the pandemic.
High hopes hang on the small vials of liquid on show at a Beijing trade fair this week -- vaccine candidates produced by Chinese companies Sinovac Biotech and Sinopharm.
Neither has hit the market yet but the makers hope they will be approved after all-important phase 3 trials as early as year-end. — AFP/Beiyi Seow
The Trump administration has urged US states to get ready to distribute a potential Covid-19 vaccine by November 1, media reported Wednesday, in the latest sign of the accelerating race to deliver a vaccine by year's end.
"CDC urgently requests your assistance in expediting applications for these distribution facilities," read a letter from Robert Redfield, director of the Centers for Disease Control and Prevention, quoted by The Wall Street Journal.
The CDC, "if necessary, asks that you consider waiving requirements that would prevent these facilities from becoming fully operational by Nov. 1, 2020," two days before the US presidential election, the letter said. — AFP
Hong Kong launches a mass coronavirus testing scheme, but calls for millions to take up the offer have been undermined by deep distrust of the government following China's crushing of the city's democracy movement.
The free voluntary tests are part of an attempt to stamp out a third wave of infections that began in late June and saw the densely populated city reimpose economically painful social distancing measures.
But the programme has been hampered by a limited response due to the involvement of mainland Chinese testing firms and doctors -- and swirling public fears of the harvesting of data and DNA as Beijing cracks down on calls for democratic reform. — AFP
Hong Kong launched a mass coronavirus testing scheme on Tuesday, but calls for millions to take up the offer have been undermined by deep distrust of the government following China's crushing of the city's democracy movement.
The free voluntary tests are part of an attempt to stamp out a third wave of infections that began in late June and saw the densely populated city reimpose economically painful social distancing measures.
But the program has been hampered by a limited response due to the involvement of mainland Chinese testing firms and doctors — and swirling public fears of the harvesting of data and DNA as Beijing cracks down on calls for democratic reform.
Since registration began on Saturday, 510,000 people have signed up — around seven percent of the city's 7.5 million population. — AFP
The head of the US Food and Drug Administration raises the possibility that a future vaccine against the coronavirus might be given emergency approval before the end of trials designed to ensure its safety and effectiveness. — AFP
Anthony Fauci, the United States' top infectious diseases official, says the government wouldn't make any future COVID-19 vaccine obligatory for the general public -- though local jurisdictions could make it mandatory for some groups, like children.
"You don't want to mandate and try and force anyone to take a vaccine. We've never done that," says Fauci, a member of the White House coronavirus task force, during a video talk organized by George Washington University.
"You can mandate for certain groups of people like health workers, but for the general population you can't" he added, citing the example of the National Institutes of Health, where health workers can't treat patients without a flu shot. — AFP
Mexican President Andres Manuel Lopez Obrador said Monday that he would be among the first to receive a Russian coronavirus vaccine if it is shown to be effective.
Russia's announcement last week that it was the first in the world to approve a coronavirus vaccine was met with caution from Western scientists who said it still needed to be proved safe and effective.
"I would be the first to get vaccinated, because it matters a lot to me, but we have to ... ensure that it's something effective and that it's available to everyone," Lopez Obrador said at his daily news conference. — AFP
Mexico and Argentina aim to have a coronavirus vaccine available for Latin America early next year under a production agreement with drugs giant AstraZeneca, the Mexican government said Thursday.
The vaccine, being developed in collaboration with the University of Oxford, is one of the most promising of dozens that researchers around the world are racing to prove safe and efficient.
The goal is to "start manufacturing to have the vaccine in the first quarter of next year," Mexican President Andres Manuel Lopez Obrador told a news conference. — AFP
The Brazilian state of Parana signed a deal Wednesday to test and produce Russia's new coronavirus vaccine, though officials stressed they would have to be sure of its safety and effectiveness first.
The vaccine would have to receive Brazilian regulatory approval and complete Phase 3 clinical trials, or large-scale testing in humans, before being produced in Brazil, said officials from the southern state.
Production, if it goes ahead, would likely only start in the second half of 2021, said Jorge Callado, head of the state-run Parana Technology Institute, which signed the deal with the Russian Direct Investment Fund. — AFP
Palace spokesperson Harry Roque says the earliest time that President Rodrigo Duterte can get vaccinated with the Russian vaccine is on May 1, 2021. — The STAR/Alexis Romero
Following Russia's announcement that it has supposedly developed a COVID-19 vaccine, the United States says it is not "a race to be first."
Noting that the Russian vaccine is now only beginning, US Health and Human Services Secretary Alex Azar says two of the six vaccines that the American government invested in entered the third phase of clinical trials weeks ago.
"The data from the initial trials in Russia have not been disclosed, it’s not transparent," Azar tells reporters in a teleconference.
US President Donald Trump announces a $1.5 billion contract with US biotech company Moderna for 100 million doses of an eventual coronavirus vaccine, the sixth such deal reached since May.
"I'm pleased to announce that we have reached an agreement with Moderna to manufacture and deliver 100 million doses of their coronavirus vaccine candidate," Trump says at a White House news conference. "The federal government will own these vaccine doses, we're buying them."
"We're on track to rapidly produce 100 million doses as soon as the vaccine is approved, and up to 500 million shortly thereafter, so we'll have 600 million doses," he adds. — AFP
The World Health Organization says any WHO stamp of approval on a COVID-19 vaccine candidate would require a rigorous safety data review, after Russia announced Tuesday it had approved a vaccine.
President Vladimir Putin said Russia had become the first country to approve a vaccine offering "sustainable immunity" against the new coronavirus.
"We are in close contact with the Russian health authorities and discussions are ongoing with respect to possible WHO pre-qualification of the vaccine," the United Nations health agency's spokesman Tarik Jasarevic tells reporters in Geneva at an online press briefing. — AFP
President Donald Trump says that a vaccine may be produced ahead of the US presidential election on November 3 — a more optimistic timeline than given by his top infectious diseases doctor.
Asked by radio talk show host Geraldo Rivera whether a vaccine could come by the election, Trump says: "I think in some cases, yes, possible before. But right around that time."
Trump says the vaccine would be ready "sooner than the end of the year. Could be much sooner."
"We have a lot of vaccines under study by the way. We look like we're going to be really good on vaccines and therapeutics also," he says.— AFP
The Armed Forces of the Philippines welcomes the plan of President Rodrigo Duterte for the military to manage the distribution of COVID-19 vaccine once it is abailable.
Maj. Gen. Edgard Arevalo, spokesperson of the AFP, says the AFP chief has directed his staff to plan the task.
"Bagaman at kababanggit pa lang ng Pangulo ang tungkol sa bagay na yan, kaagad pong inatasan ng ating AFP Chief of Staff General Felimon Santos Jr. ang kanyang mga staff upang pagpaplanuhan at paghandaan yan before December kung kailan pinaniniwalaan na magkakaroon na ng vaccine para sa COVID-19," Arevalo says.
Finance Secretary Carlos Dominguez III says the Philippines will allocate around P20 billion to purchase COVID-19 expected to be available by "December."
Four vaccines from China, the United Kingdom and the United States are being looked at. The Department of Health will evaluate which is best to use.
"Vaccines will be for free to 'poorest of the poor'," Domingue said, adding that the Philippines targets to vaccinate 20 million people once COVID-19 vaccine is available. That's nearly a fifth of the 108 million people. — Philstar.com/Prinz Magtulis
Johnson & Johnson's announces that its lead vaccine candidate elicited a robust immune response as demonstrated by "neutralizing antibodies" in pre-clinical studies.
The data, published in journal Nature, showed that the company’s adenovirus serotype 26 nickname Ad26 vaccine successfully prevented subsequent infection and completely protected the lungs from the SARS-CoV2 virus in non-human primates in a single dose during the pre-clinical study.
The pre-clinical tests were conducted by researchers from Beth Israel Deaconess Medical Center in collaboration with the Janssen Pharmaceutical Companies of Johnson & Johnson and others as part of its ongoing collaboration to accelerate the development of a SARS-CoV-2 vaccine.
Pharma giants Sanofi and GSK said on July 29, 2020, that they have agreed to supply Britain with up to 60 million doses of a potential COVID-19 vaccine. The agreement covers a vaccine candidate developed by France's Sanofi in partnership with the UK's GSK and is subject to a "final contract."
This thread collects some of the major developments in the search for a vaccine to ease the new coronavirus pandemic. (Main photo by AFP/Joel Saget)
- Latest
- Trending





























