Dengvaxia scandal haunts Philippines' COVID-19 vaccine rush

MANILA, Philippines — A global scramble to find the first coronavirus disease-2019 (COVID-19) vaccine has arrived in the Philippines from Russia, triggering concerns the rush to develop a convincing defense against the deadly disease is creating more questions than answers.
Western scientists have already warned against administering Sputnik V, the Russian government-funded vaccine against the deadly COVID-19, without undergoing the crucial third phase of typical vaccine development that gauges the vaccine’s efficacy on a broader population. In Manila, stakes are higher coming off from a botched dengue vaccination program that spawned into a bigger political controversy just three years ago.
“People are concerned about a repeat of Dengvaxia and for good reasons,” said Dr. Beaver Tamesis, president of the Pharmaceutical and Healthcare Association of the Philippines Inc., an industry group.
“There is this lack of information about the vaccine that is concerning the public,” he said in a phone interview.
At the Palace, presidential spokesperson Harry Roque was quick to qualify the Duterte administration’s early optimism for a vaccine this year. As it is now turning out, Sputnik V may not be ready for deployment until May next year, when President Rodrigo Duterte himself is expected to be vaccinated first as he promised, following the third phase of trials. Part of those trials will be held in the country from October 2020 to March 2021.
There are many concerns about Sputnik V, much more than the fact that it holds a name similar to a local street gang. Even in the local pharmaceutical industry, Tamesis said few things are known about the vaccine, details on which have not been published but already registered by Russia as a vaccine.
“It’s not so much about that they approved it, or who approved it, but that they approve it despite the fact no publication or database was shared,” Tamesis explained.
That said, Rolando Enrique Domingo, director-general of Food and Drugs Administration, assured the public the government will study the COVID-19 vaccines carefully. “We will not accept an application for approval unless the Phase III trial is completed,” he said in a text message. Health Secretary Francisco Duque III did not respond to request for comment.
Dengvaxia effect
The health risks to an unviable vaccine are high and, in very rare cases, can potentially lead to death. Tony Leachon, formerly special adviser to the Philippines’ COVID-19 task force, said “lots of things are possible” post-vaccination, adverse effects which will only be clearer under the third phase of clinical trials where 3,000 Filipinos are expected to volunteer and participate.
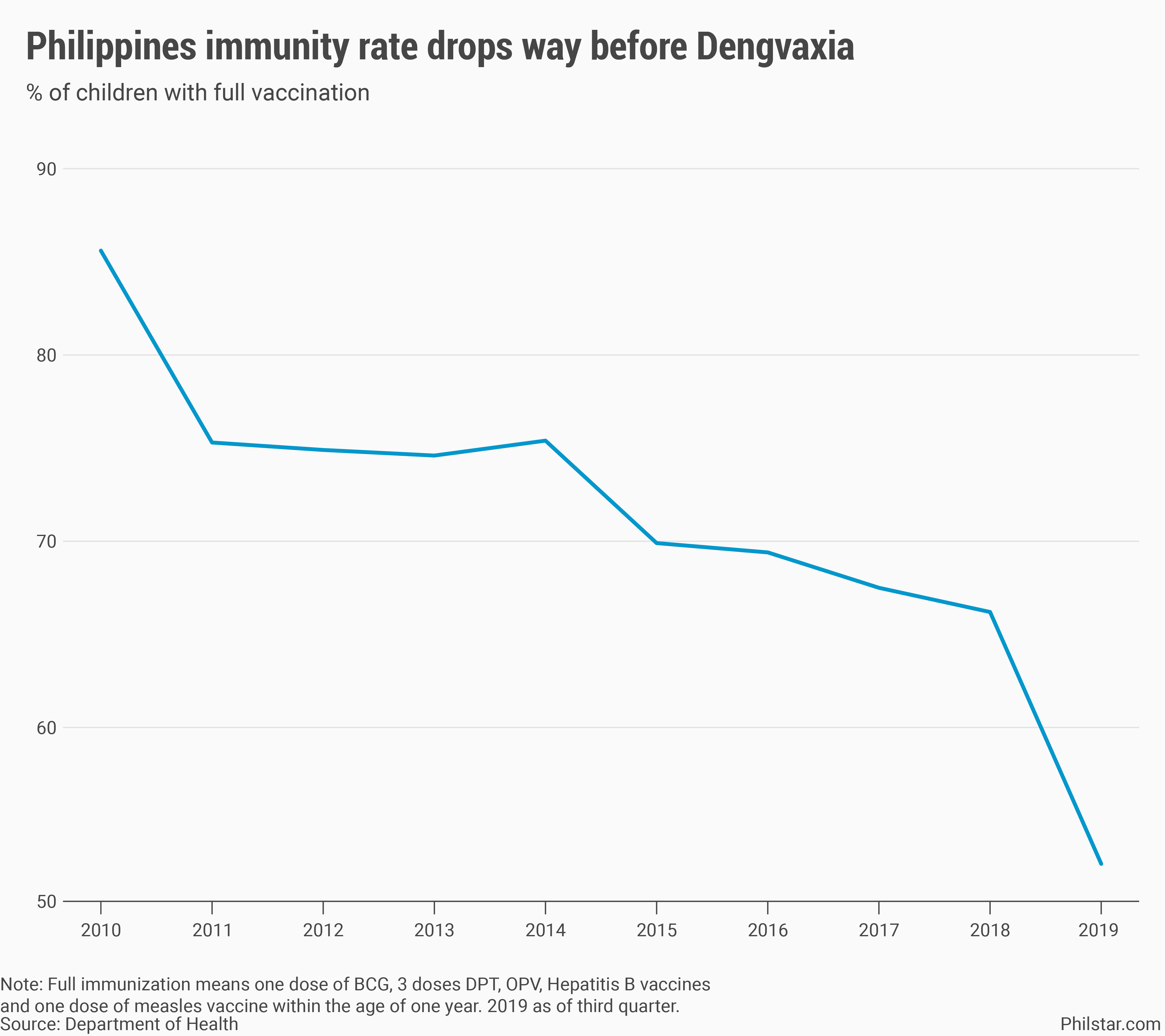
But risks go beyond the vaccine’s potential side effects. In the Philippines, the Dengvaxia controversy in 2017 only accelerated public distrust even to vaccines long proven to be effective on diseases like polio and measles, which have since resurfaced. Health data show that from as high as 85.6% of child population in 2010, full immunization rates dropped to 66.2% in 2018.
As of the third quarter of 2019, full immunization went down further to 52.7%.
Tamesis said government has a time-tested public immunization program that can be tapped as soon as a COVID-19 vaccine is available, but that will only be effective if people would trust the vaccine. “There should be willingness to come and have the vaccine,” he said.
Dr. Benjamin Co, an infectious disease expert, agreed. “I think people are not desperate for the vaccine considering that the disease presents mildly than severe. So the rush to a vaccine is more of personal and economic reasons as these have been disrupted,” Co said in an online exchange.
Beyond health, repercussions can be catastrophic, especially for vaccines for a disease that has spiraled into pandemic. After Dengvaxia hearings in Congress, where vaccine procurement was scrutinized, the health department became more hesitant to enter into public contracts for essential medicines, distributed for free to poor families in government clinics.
While medicine budgets were increased to P15.4 billion in 2019 from P7.89 billion in 2015, budget data showed actual spending plummeted to just 11.3% of funds appropriations in 2018, the latest period on which data is available. “Slowly now, we are seeing some recovery from the glacial pace of procurement. It’s still a little slow, but at least purchase orders are coming,” Tamesis said, adding it could also be a result of COVID-19 emergency.
In a broader scale, growth in public health expenditure was likewise stunted by Dengvaxia. Government data showed that in 2018, a year after the probe, health disbursements by national government dropped 7.3% year-on-year, with outlays from health department shrinking a faster 9.5% annually. As a result, Filipinos had to shell out more for their health, shouldering 53.9% of health costs that year, up from 53% in 2017.
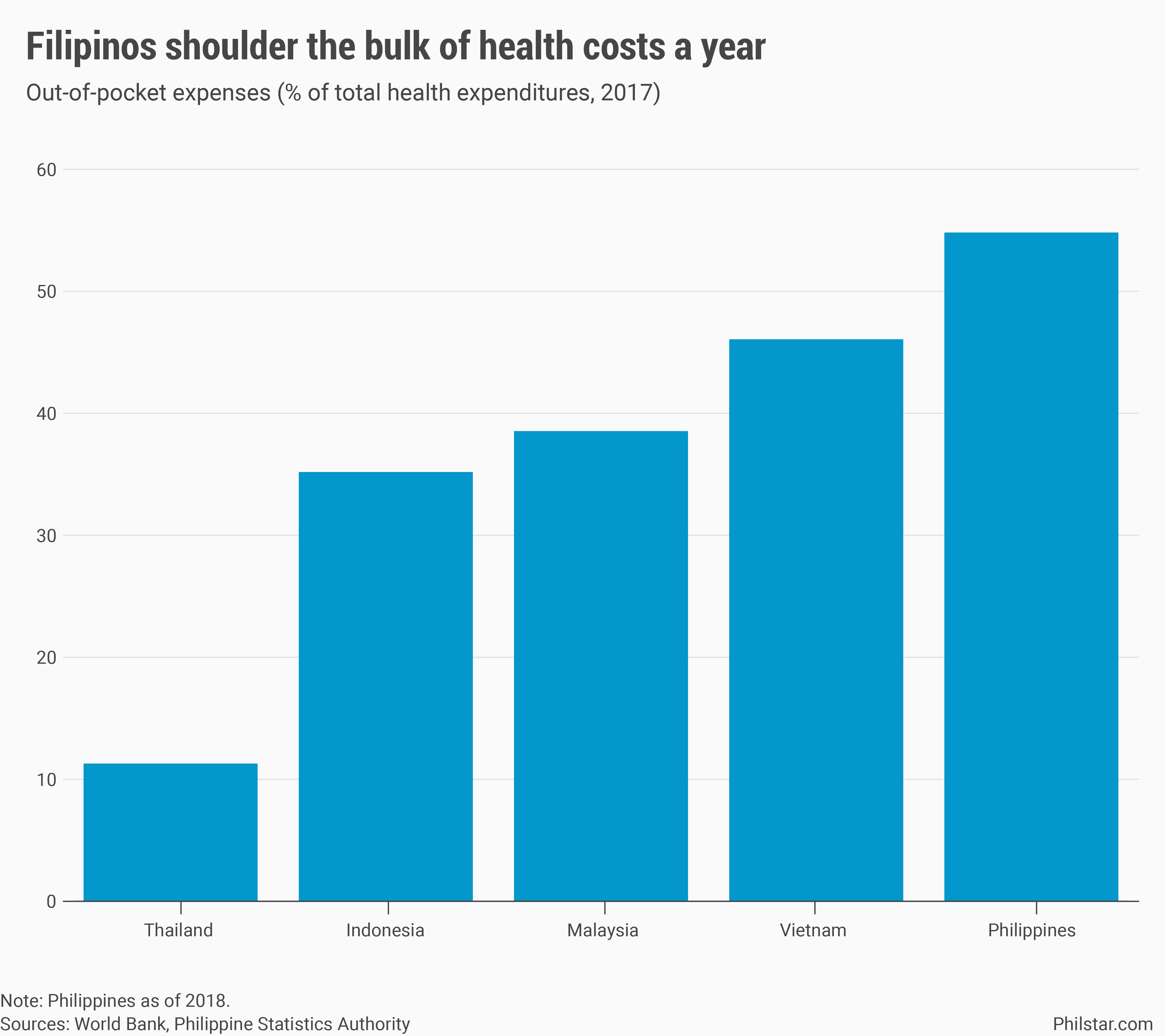
The Duterte administration targets to reduce out-of-pocket spending to 50% by 2022, albeit still the highest in Southeast Asia, but that goal is now in jeopardy mostly because of how the coronavirus has gone beyond state control. But also due to a P15-billion corruption scandal that has rocked Philippine Health Insurance Corp., the agency tasked to enforce Duterte’s centerpiece universal healthcare program.
Ultimately, Dengvaxia remains in the market— just not in the Philippines— and is considered effective against dengue for people who contracted the disease in the past. That said, events that led to the revocation of the vaccine's local license in 2019 demonstrate what happens when public health is politicized, and the risk of that happening in COVID-19 is high.
"(Dengvaxia) just changed its label after two years. Every drug will have that hiccup. It's called post-market surveillance...It's only when there are more patients will we see the true side effects of any drug or vaccine," Co said.
"I will always wait for a better medicine or vaccine before advocating it," he said.
From 2 years to 6 months
The science and technology department assured the public of a thorough study of Sputnik V, but the government’s timeline for third phase of trials does not invite confidence. Testing will be accelerated from the typical 2 to 3 years to just 6 months. To compare, Sanofi Pasteur Inc., the French pharmaceutical firm that developed Dengvaxia, spent 25 months for their Phase III testing.
“The question now shifts as to how long will you have to wait to conclude the Phase III of clinical trials. What you need to see is what happens down the line three, six months or even a year after. Will there be side effects?” Tamesis said.
To be fair, Co said a vaccine’s efficacy and safety provisions, even when already in market, is consistently reevaluated and updated. In the end, it is up to health officials to carefully evaluate whether the vaccine is safe for public usage with the information they have at hand.
“There are many ways to accelerate it (Phase III). For example, if the manufacturer is sure of its product, it can start manufacturing already while they are still in clinical trials,” he said.
“If Phase III isn’t successful, then it goes back to square one and many drugs had that happen to them,” he added.
- Latest
- Trending